What is 1,1,1,3,3,3-Hexafluoro-2-propanol?
Dec 30,2019
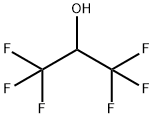
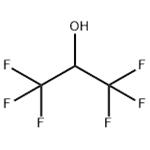
1,1,1,3,3,3-Hexafluoro-2-propanol also known as hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound with the formula (CF3)2CHOH. This fluoroalcohol finds use as solvent and synthetic intermediate. It appears as a colorless, volatile liquid that is characterized by a strong, pungent odor. As a solvent hexafluoro-2-propanol is polar and exhibits strong hydrogen bonding properties enabling it to dissolve substances that serve as hydrogen-bond acceptors, such as amides and ethers. Hexafluoro-2-propanol is transparent to UV light with high density, low viscosity and low refractive index [1].
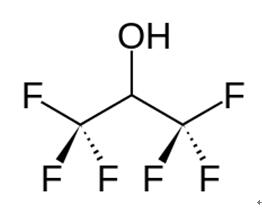
1,1,1,3,3,3-Hexafluoro-2-propanol is an organofluorine compound formed by substitution of all the methyl protons in propan-2-ol by fluorine. It is a metabolite of inhalation anesthetic sevoflurane. It has a role as a drug metabolite. It is an organofluorine compound and a secondary alcohol. It derives from a propan-2-ol. 1,1,1,3,3,3-hexafluoro-2-propanol is a clear colorless oily liquid with an aromatic odor. (NTP, 1992) Combustible, may cause burns to skin, eyes and mucous membranes [2].
1,1,1,3,3,3-Hexafluoro-2-propanol is prepared from hexafluoropropylene through hexafluoroacetone, which is then hydrogenated [3].
(CF3)2CO + H2 → (CF3)2CHOH
1,1,1,3,3,3-Hexafluoro-2-propanol is a speciality solvent for some polar polymers and organic synthesis. It is especially effective for solubilizing a wide range of polymers, including those that are not soluble in the most common organic solvents, such as: polyamides, polyacrylonitriles, polyacetals, polyesters (e.g. polyglycolide), and polyketones. It has also found use in biochemistry to solubilize peptides and to monomerize β-sheet protein aggregates. Because of its acidity (pKa = 9.3), it can be used as acid in volatile buffers for ion pair HPLC - mass spectrometry of nucleic acids [4].
1,1,1,3,3,3-Hexafluoro-2-propanol is both the precursor and the chief metabolite of the inhalation anesthetic sevoflurane.
1,1,1,3,3,3-Hexafluoro-2-propanol is a volatile, corrosive liquid that can cause severe burns and respiratory problems.
1,1,1,3,3,3-Hexafluoro-2-propanol is a polar solvent of high ionizing power and facilitates Friedel–Crafts-type reactions, using covalent reagents in the absence of a Lewis acid catalyst. 1,1,1,3,3,3-Hexafluoro-2-propanol clusters catalyzes the epoxidation of cyclooctene and 1-octene with hydrogen peroxide.
1,1,1,3,3,3-Hexafluoro-2-propanol was used for preparing hexafluoroalcohol-functionalized methacrylate polymers for lithographic/nanopatterning materials [5].
References
[1] https://en.wikipedia.org/wiki/Hexafluoro-2-propanol
[2] https://pubchem.ncbi.nlm.nih.gov/compound/1_1_1_3_3_3-Hexafluoro-2-propanol
[3] Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” in Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, 2007. doi:10.1002/14356007.a11_349
[4] Apffel, A.; Chakel, J.A.; Fischer, S.; Lichtenwalter, K.; Hancock, W.S. (1997). "Analysis of oligonucleotides by HPLC-electrospray ionization mass spectrometry". Anal. Chem. 69: 1320–1325. doi:10.1021/ac960916h
[5] https://www.sigmaaldrich.com/catalog/product/aldrich/105228?lang=en®ion=US
- Related articles
- Related Qustion
- The wide range of applications: 1,1,1,3,3,3-Hexafluoro-2-propanol Apr 26, 2024
Hexafluoro-2-propanol (HFIP; 1,1,1,3,3,3-Hexafluoro-2-propanol) is a low-boiling solvent used in a variety of chemical applications.
- 1,1,1,3,3,3-Hexafluoro-2-propanol: Application, synthesis, toxicity and environmental hazard May 17, 2023
1,1,1,3,3,3-Hexafluoro-2-propanol also known as hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound finds use as solvent and synthesis.
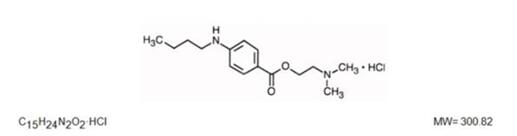
Tetracaine hydrochloride is a local anesthetic used to numb the eyes, nose, or throat. It may also be used before starting an intravenous to decrease pain from the procedure. Typically it is applied as a liquid to the area.....
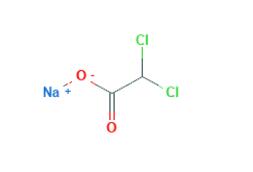
Dec 30,2019AnestheticsSodium Dichloroacetate is the sodium salt of dichloroacetic acid with potential antineoplastic activity. Dichloroacetate ion inhibits pyruvate dehydrogenase kinase, resulting in the inhibition of glycolysis.....
Dec 30,2019Organometallic compounds1,1,1,3,3,3-Hexafluoro-2-propanol
920-66-1You may like
1,1,1,3,3,3-Hexafluoro-2-propanol manufacturers
- 1,1,1,3,3,3-Hexafluoro-2-propanol
-
- 2025-12-13
- CAS:920-66-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Mung bean peptide
-

- $0.00 / 1KG
- 2025-12-13
- CAS:
- Min. Order: 1KG
- Purity: 75% HPLC
- Supply Ability: 1000KG
- 1,1,1,3,3,3-Hexafluoro-2-propanol
-

- $0.00 / 200kg
- 2025-12-12
- CAS:920-66-1
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 500mt