4-Pyridinecarboxaldehyde: Versatility in Medicinal Chemistry and its Preparation Method
May 11,2024
General Description
4-Pyridinecarboxaldehyde is a yellow-brown liquid utilized in organic synthesis. It is stored under inert gas due to its sensitivity to air and humidity. In medicinal chemistry, it shows promise in treating idiopathic pulmonary fibrosis (IPF) and as an antimalarial agent. The compound is prepared through oxidation of 4-pyridinemethanol or transformation of 4-cyanopyridine, with both methods providing high yields. Its versatility and ability to modulate biological targets make it a valuable building block for developing therapeutic compounds. Overall, 4-Pyridinecarboxaldehyde holds significant potential for future drug development efforts.

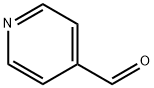
Figure 1. 4-Pyridinecarboxaldehyde
Overview
4-Pyridinecarboxaldehyde, commonly known as pyridine-4-formaldehyde, is a chemical compound with the molecular formula C6H5NO. This yellow-brown liquid exhibits a distinct chemical structure, characterized by a pyridine ring with an aldehyde group attached to the 4-position. With a melting point ranging from -4°C to -2°C and a boiling point of 71-73°C at 10 mm Hg, it is sensitive to air and humidity, requiring storage under inert gas at 2-8°C. Used primarily in organic synthesis, 4-Pyridinecarboxaldehyde is classified as a hazardous substance due to its irritant properties, requiring special handling and packaging for safe transportation. 1
Versatility in Medicinal Chemistry
4-Pyridinecarboxaldehyde, a versatile compound in medicinal chemistry, finds applications in various therapeutic areas. One notable application is in the treatment of idiopathic pulmonary fibrosis (IPF). In a recent study, researchers investigated the role of HDAC6, an enzyme associated with IPF progression. By synthesizing a series of novel hHDAC6 inhibitors, with 4-Pyridinecarboxaldehyde as a key component, they aimed to develop potent pharmacological tools for IPF treatment. These inhibitors exhibited low inhibitory potency over other HDAC enzymes, such as hHDAC1 and hHDAC8, reducing the risk of off-target effects. Structural analysis and structure-activity relationship studies aided in optimizing the binding mode of these molecules, enhancing their efficacy in inhibiting fibrotic sphere formation and cell viability associated with IPF. Furthermore, the efficacy of the most promising analogs, including 4-Pyridinecarboxaldehyde derivatives, was validated in human lung models, demonstrating their potential in reversing the IPF phenotype. In another study, 4-Pyridinecarboxaldehyde derivatives were explored for their antimalarial properties. Through phenotypic whole-cell screening, compounds with potent anti-malarial activity against multiple resistant strains of Plasmodium falciparum were identified. Structure-activity studies elucidated relationships between potency and specific structural modifications, addressing issues such as aqueous solubility and metabolic vulnerability. Analogues with structural modifications showed excellent anti-malarial activity in vivo, paving the way for further preclinical studies. Overall, 4-Pyridinecarboxaldehyde serves as a valuable building block in the design and synthesis of compounds with therapeutic potential in challenging diseases like IPF and malaria. Its versatility and ability to modulate biological targets make it a promising candidate for future drug development efforts. 2
Preparation Method
4-Pyridinecarboxaldehyde, a valuable intermediate in organic synthesis, can be prepared via two distinct methods: oxidation of 4-pyridinemethanol and transformation of 4-cyanopyridine. For the oxidation method, 4-pyridinemethanol undergoes oxidation or dehydrogenation in the presence of oxygen and catalytic system 1-methylimidazole, 9-Azabicyclo[3.3.1]non-9-yloxy copper bromide supported on TentaGel resin. The reaction is conducted in acetonitrile solvent at room temperature, followed by filtration, solvent removal, and analysis by NMR spectroscopy. This process achieves a high yield of 4-pyridinecarboxaldehyde. Alternatively, 4-cyanopyridine can be transformed into 4-pyridinecarboxaldehyde via reduction using potassium carbonate, water, and carbon catalyst in a mixed solvent of dimethyl sulfoxide and water. The reaction is conducted in a closed high-pressure reaction tube at 60°C for 8 hours. After separation of the catalyst and organic phase, the resulting product is analyzed using GC-MS. Both methods offer efficient routes to produce 4-pyridinecarboxaldehyde, a versatile compound widely used in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals. These methods provide high yields and can be adapted for large-scale production. 3
Reference
1. 4-Pyridinecarboxaldehyde. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 13389。
2. Campiani G, Cavella C, Osko JD, et al. Harnessing the Role of HDAC6 in Idiopathic Pulmonary Fibrosis: Design, Synthesis, Structural Analysis, and Biological Evaluation of Potent Inhibitors. J Med Chem. 2021; 64(14): 9960-9988.
3. Wang K. Metal-free nitrogen -doped carbon nanosheets: a catalyst for the direct synthesis of imines under mild conditions†. Green Chemistry. 2019; 9: 2448–2461.
- Related articles
- Related Qustion
- Application research of 4-pyridinecarboxaldehyde Nov 14, 2025
4-Pyridinecarboxaldehyde was shown to be an efficient building block for synthesis of Schiff bases,of which related research and application were introduced.
- 4-Pyridinecarboxaldehyde: properties and applications in various fields Sep 19, 2023
4-Pyridinecarboxaldehyde is a yellow liquid with a pungent odor, used in biosensors and corrosion protection materials.
- 4-Pyridinecarboxaldehyde-Application Dec 20, 2019
4-Pyridinecarboxaldehyde is a colorless oily liquid with a distinctive odor. Older samples are often brown-colored owing to impurities.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThymine's critical role in DNA stability and protein synthesis influences genetic studies and bacterial physiology, including thymine-less death and potential implications for cancer research.....
May 11,2024API4-Pyridinecarboxaldehyde
872-85-5You may like
4-Pyridinecarboxaldehyde manufacturers
- 4-Pyridinecarboxaldehyde
-

- $10.00 / 1KG
- 2025-12-11
- CAS:872-85-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 4-Pyridinecarboxaldehyde
-
- $0.00 / 25kg
- 2025-12-01
- CAS:872-85-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- 4-Pyridinecarboxaldehyde
-

- $10.00 / 1KG
- 2025-05-26
- CAS:872-85-5
- Min. Order: 1KG
- Purity: 99.%
- Supply Ability: 10 ton