Ethyl (2,4,6-trimethylbenzoyl) Phenylphosphinate: Characteristics, Preparation Methods and Difference with TPO
Feb 27,2024
General Description
Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate (TPO-L) is a highly efficient yellow liquid photoinitiator used in UV polymerization, particularly effective in curing low yellowing systems like white coatings. With a molecular weight of 316.3 and purity over 99.0%, it has a flash point of 242.8°C and minimal volatile matter. Its synthesis involves controlled reactions to produce a high-purity product suitable for various UV-curable applications. Contrasted with traditional TPO initiators, Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate offers improved reactivity profiles for enhanced surface and through-cure, making it a valuable component in UV-curable formulations for diverse industrial uses.

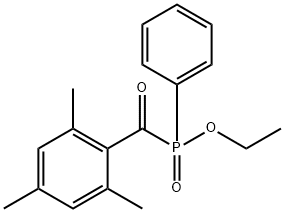
Figure 1. Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
Characteristics
Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate, or TPO-L, is a yellow liquid photoinitiator widely used in the UV polymerization and curing of resins. Its high efficiency and free radical type I nature make it suitable for various applications. Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate is especially effective in curing low yellowing, white systems, and thick film coatings. It is also utilized in screen printing inks, offset printing inks, flexographic inks, and wood coatings. With a molecular weight of 316.3, Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate exhibits a content of at least 99.0%, ensuring its high purity. It has a flash point of 242.8°C, a refractive index of 1.549, and a density of 1.15g/cm3. The volatile matter present in Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate is minimal, not exceeding 0.5%. The absorption wavelengths of 299nm and 366nm allow for efficient initiation of polymerization under UV light. Overall, Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate's characteristics, including its appearance, stability, and specific absorption wavelengths, make it an excellent choice for the formulation of UV-curable materials across various industrial and commercial sectors. 1
Preparation methods
The synthesis of Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate involves a two-step process. First, 3,5-dimethylbenzoylphenol is reacted with trimethylphosphinyl chloride under controlled conditions to obtain the desired product. This initial reaction is conducted in a dry organic solvent under an inert atmosphere, with careful temperature control during the addition of trimethylphosphinyl chloride. Once the reaction is complete, the resulting mixture is diluted with water, and the product is extracted and concentrated to isolate Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate. Following this, in the Arylphosphine oxide section, the synthesis continues by introducing ethyl diphenylphosphonite into a high-level dripping tank under negative pressure. This compound is then added dropwise to a mixture of 2,4,6-trimethylbenzoyl chloride and triethylamine in the acylphosphine oxygen synthesis kettle at a specific temperature. The reaction proceeds for about 2 hours, with the generation of chloroethane gas being managed carefully. To further process the reaction mixture, dilute hydrochloric acid is added to remove residual acid chloride, followed by washing the solution with toluene, alkaline water, acid water, and neutral water in sequence. The organic phase is then subjected to vacuum desolventization to recover the solvent. Decolorization of the product is achieved by heating with activated carbon, filtration, and ethanol removal, resulting in the final product of Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate with a purity exceeding 96%. Overall, the synthesis of Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate involves precise control of reaction conditions, sequential processing steps, and purification techniques to yield a high-purity product suitable for various applications in UV polymerization and curing processes. 2
Difference with TPO
Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate and TPO initiators are both widely used in the field of photopolymerization, but they have distinct differences in terms of chemical structure and application. Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate is a phosphine oxide photoinitiator that exhibits high reactivity upon exposure to UV light. It is commonly used in UV-curable systems such as inks, coatings, and adhesives. Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate possesses a more balanced reactivity profile compared to traditional TPO initiators, making it suitable for formulations requiring improved surface cure and through-cure. On the other hand, TPO initiators generally refer to a group of compounds, including TPO-L, that contain the 2,4,6-trimethylbenzoyl moiety and a phosphine oxide group. These initiators are known for their ability to generate free radicals upon exposure to UV light, which can initiate the polymerization of multifunctional monomers and oligomers. In summary, while Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate is a specific type of phosphine oxide photoinitiator with unique reactivity characteristics, TPO initiators represent a broader category of compounds with similar chemical features and photopolymerization capabilities. 1,3
Reference
1. Ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 16205480.
2. Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate synthesis. CN112778360, 2021, 75-76.
3. Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 166480.
- Related articles
- Related Qustion
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate: applications and toxicology Aug 10, 2023
Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate is used in shape-memory polymers and 3D printing. Low toxicity, avoid skin/eye contact for safety.
- 2,4,6-trimethylbenzoylphenylphosphinic acid ethyl ester- Reaction / Application on synthetic works Nov 19, 2019
2,4,6-Trimethylbenzoylphenylphosphinic acid ethyl ester is a photoinitiator for UV curable coatings. It is also an important organic intermediate to synthetize substituted benzoylphenylphosphinic products.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringTriadimefon controls plant diseases and promotes growth, but presents health hazards like skin irritation. Safety measures are essential.....
Feb 27,2024APIYou may like
Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate manufacturers
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate/TPO-L
-

- $0.00 / 25kg
- 2025-12-13
- CAS:84434-11-7
- Min. Order: 1kg
- Purity: 98%min
- Supply Ability: 10 tons
- Photoinitiator TPO-L
-

- $0.00 / 25Kg/Drum
- 2025-12-12
- CAS:84434-11-7
- Min. Order: 1KG
- Purity: 95%
- Supply Ability: 500mt/year
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
-
- $10.00 / 1KG
- 2025-12-11
- CAS:84434-11-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt