Introduction and Preparation of Amphotericin B
Aug 9,2022
General description
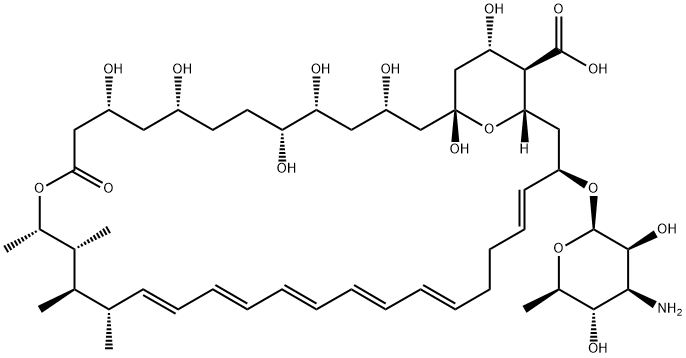
Amphotericin B (AmB) is a polyene macrolide antibiotic produced by Streptomyces nodosus and is a medically important broad-spectrum antifungal drug. AmB has a molecular formula of C47H73NO17 and a molecular weight of 924.10 (Figure 1). It is hygroscopic and is easily damaged under light. It gradually turns black when heated, and decomposes when it exceeds 170°C. Pure AmB is an orange-yellow needle or columnar crystal, odorless and tasteless, insoluble in water, absolute ethanol, ether, benzene and toluene, slightly soluble in acid dimethylformamide (DMF), and soluble in dimethyl sulfoxide (DMSO) [1].
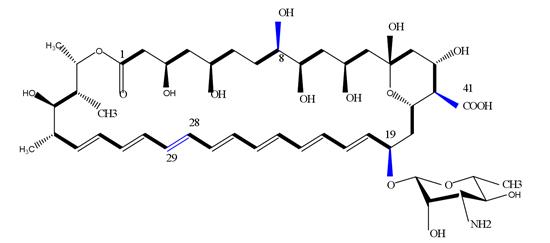
Figure 1. The molecular formula of Amphotericin B
During the biosynthesis of amphotericin B, a co-metabolite Amphotericin A (AmA) with similar structure and low antibacterial activity will be produced. The only difference lies in the double bond between C28-C29, among which AmB is an unsaturated bond, while AmA is a saturated bond [2]. The similarity in structure increases the difficulty of the later separation and purification of Amphotericin B.
Application and Mechanism of Action [3-5]
As a medically important broad-spectrum antifungal drug, Amphotericin B is a polyene macrolide antibiotic produced by Streptomyces nodosus. Clinically, it mainly acts on most fungi such as Cryptococcus neoformans, Histoplasma, Cyclospora, Candida, Absidia, Endomys and some Aspergillus. Although AmB has been on the market for more than 50 years, and with the continuous development and promotion of various newly developed antifungal drugs, due to its medicinal value and clinical importance, AmB is still irreplaceably widely used as a treatment for systemic fungal infections. antibiotics, considered the "gold standard" for the treatment of many severe deep fungal infections.
As a polyene antifungal drug, the main mechanism of action of amphotericin B is that after the polyene binds with sterols (such as ergosterol) on the sensitive fungal cell membrane to form a channel, it damages the permeability of the cell membrane and causes intracellular damage. Some important substances such as potassium ions, nucleotides, amino acids, etc. leak out, thereby destroying the normal metabolism of cells, inhibiting the growth of fungi, and eventually leading to cell death. It is this unique antibacterial mechanism that prevents fungi from developing resistance and is the last line of defense against systemic fungal infections. In addition to its antifungal properties, amphotericin B also exhibits many other biological activities such as antiviral, antiparasitic, etc., such as inhibiting human immunodeficiency virus (HIV) infection of cultured cells, activating HIV-specific macrophages Cells, functions such as delaying the onset of viral disease symptoms in animal models, and at the same time having anti-parasitic activity against Leishmania, clinically used for the treatment of cutaneous leishmaniasis. Although amphotericin B inhibits the selective toxicity of fungal cells, it has many serious side effects, especially nephrotoxicity, neurotoxicity and cardiotoxicity. Amphotericin B is still widely used. Chemical modification can also produce biologically active derivatives with increased water solubility and reduced toxicity, such as amphotericin B liposomes. With the in-depth understanding of the side effects of amphotericin B, liposome-encapsulated amphotericin B came into being. After encapsulation, amphotericin B is taken up through the liver and slowly released into the blood, avoiding direct organ damage. , The incidence of adverse reactions of amphotericin B liposomes was significantly decreased. Mucormycosis has had a high mortality rate in the past. Early application of antifungal drugs and early excision of infected necrotic tissue is the key to improving the cure rate. The first-line drug is amphotericin B. Candida infection is the most common clinical fungal infection, and the combination of amphotericin B and flucytosine in the treatment of Candida infection has been used clinically for many years.
Preparation
Amphotericin B currently on the market is mainly prepared by biological fermentation. The main industrial Amphotericin B production strain is Streptomyces nodosus, but the strain will also produce AmA with low antibacterial activity and toxicity in the production process. At present, the highest production level of amphotericin B in China has reached the fermentation unit of 14469 µg/mL [6]. However, due to the long fermentation cycle of this strain, the low fermentation unit of amphotericin B and the unstable industrial production process, the production cost is too high, and serious Industrial production is restricted. Therefore, research and development of new strategies are urgently needed to improve the fermentation level of amphotericin B and reduce the production cost.
Biosynthesis of Amphotericin B
In Streptomyces tuberculosis, the biosynthesis of Amphotericin B starts with 3 propionate and 16 acetate precursors as the starting synthetic units, and cyclizes to form a PKS polyketide skeleton through an 18-step extension reaction, and then through further glycosylation, carboxylation, redox and other post modification steps to form the final product Amphotericin B. The genes in the AmB biosynthetic gene cluster include: PKS genes, redox genes, post-modifier genes, transport genes, regulatory genes, and other genes [7,8]. In addition, the biosynthesis of AMB involves various key metabolic pathways in Streptomyces nodosus, including intermediate carbon metabolism, amino acid metabolism, shikimic acid pathway, etc. Figure 2 illustrates in detail the close relationship between AMB biosynthesis and different metabolic pathways from glucose, in which the key pathways are marked with red pentagons. (EMP, glycolytic pathway; PPP, pentose phosphate pathway; TCA, tricarboxylic acid cycle. G6P, glucose 6-phosphate; F6P, fructose-6-phosphate; G3P, glyceraldehyde 3-phosphate; 3PG, 3-Phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; Ace CoA, acetyl-CoA; Cit, citric acid; αKG, α-ketoglutarate; SucCoA, succinyl-CoA; Suc, succinate acid; Oxa, oxaloacetic acid; Asp, aspartic acid; Thr, threonine; Ile, isoleucine; Ala, alanine; Val, valine; Leu, leucine; 6PG, 6- Phosphogluconate; Ru5P, ribose 5-phosphate; X5P, xylulose 5-phosphate; R5P, ribose-5-phosphate; S7P, sedum heptulose-7-phosphate; E4P, erythrose-4-phosphate ; Ska, shikimic acid; CHOR, chorismate; Trp, tryptophan; PHEN, benzoic acid; Phe, phenylalanine; Tyr, tyrosine)
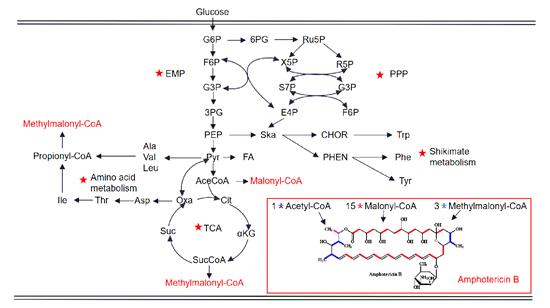
Figure 2. Schematic representation of the proposed metabolic pathways closely associated with the AmB biosynthesis in S. nodocus.
Pharmacology [9]
Amphotericin B shows a high order of in vitro activity against many species of fungi. Histoplasma capsulatum, Coccidioides immitis, Candida species, Blastomyces dermatitidis, Rhodotorula, Cryptococcus neoformans, Sporothrix schenckii, Mucor mucedo, and Aspergillus fumigatus are all inhibited by concentrations of amphotericin B ranging from 0.03 to 1.0 mcg/mL in vitro. While Candida albicans is generally quite susceptible to amphotericin B, non-albicans species may be less susceptible. Pseudallescheria boydii and Fusarium sp. are often resistant to amphotericin B. The antibiotic is without effect on bacteria, rickettsiae, and viruses.
Toxicity [10-11]
The current commercial preparation of Amphotericin B is AmB deoxycholate (DAmB, Fungizone), and its toxicity manifests as acute infusion-related reactions and dose-dependent nephrotoxicity (Figure 3). The maximum tolerated dose of AmB is 0.7–1.0 mg/kg/d, which may not be the best dose for the successful clinical treatment of invasive fungi in infected hosts. In addition, because AmB is a microbial product, its clinical use often results in infusion toxicity.
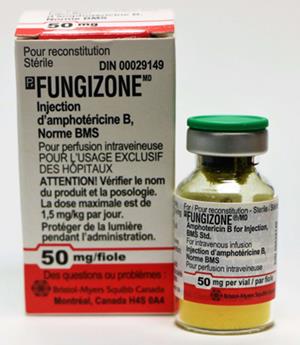
Figure 3. AmB deoxycholate, Fungizone for Injection 50 mg/Vial
AmB is recognized by Toll-like receptor (TLR) 2 and the transmembrane signaling protein CD14 on the surface of monocytes, and then induces the expression of inflammatory cytokine genes through the intracellular adaptor protein My D88 and nuclear factor B signaling pathways, resulting in Acute infusion-related toxicity. Immediately after infusion of AmB, vasoconstriction of renal arterioles results in decreased renal blood flow and decreased glomerular filtration rate. Furthermore, the binding of Am B to cholesterol in mammalian cell membranes results in non-selective destruction of mammalian cells. As LDL receptors are relatively abundant on tubular cells and HDL receptors are deficient, tubular cellular uptake of AmB is thought to be the result of LDL receptor-mediated endocytic serum LDL-AmB complex formation. After injection of AmB deoxycholate, aggregates are generated during dilution by plasma, and AmB aggregates can lead to non-selective destruction of mammalian cells, resulting in a series of toxic side effects. The higher the ratio of aggregates to monomers, the stronger the toxic side effects.
References
1. Gray KC, Palacios DS, Dailey I, et al. Amphotericin primarily kills yeast by simply binding ergosterol[J]. Proc Natl Acad Sci U SA, 2012,109(7):2234-2239.
2. Mcnamara C M, Crawforth J M, Hickman B S, et al. Biosynthesis of amphotericin B[J]. Journal of the Chemical Society Perkin Transactions, 1998, 1(1): 83-88.
3. D. Saravolatz L, Ostrosky-Zeichner L, A. Marr K, et al. Amphotericin B: Time for a new “Gold Standard”[J]. Clinical Infectious Diseases, 2003, 37(3): 415-425.
4. Jain S K, Kaur L, Abhijeet A. Safe and effective delivery of amphotericin B: A survey of patents[J]. Recent Pat Nanotechnol, 2017, 11(3): 214-234.
5. Golgher D, Vianna C H, Moura A C. Drugs against leishmaniasis: Overview of market needs and pipeline[J]. Drug Development Research, 2011, 72(6): 463-470.
6. Stiller E T, Vandeputte J, Wachtel J L. Amphotericins A and B, antifungal antibiotics produced by a Streptomycete. II. The isolation and properties of the crystalline amphotericins[J]. Antibiotics Annual, 1956, 3(4): 587-591.
7. Caffrey P, Lynch S, Flood E, et al. Amphotericin biosynthesis in Streptomyces nodosus: Deductions from analysis of polyketide synthase and late genes[J]. Chemistry and Biology, 2001, 8(7): 713-723.
8. Mcnamara C M, Crawforth J M, Hickman B S, et al. Biosynthesis of amphotericin B[J]. Journal of the Chemical Society Perkin Transactions, 1998, 1(1): 83-88.
9. National Center for Biotechnology Information. "PubChem Compound Summary for CID 5280965, Amphotericin b" PubChem.
10. Ben-Ami R, Lewis R E, Kontoyiannis D P. Immunocompromised hosts: immunopharmacology of modern antifungals.[J] Clinical infectious diseases, 2008,47(2): 226-235.
11. Laniado-Laborín R, Cabrales-Vargas M N. Amphotericin B: side effects and toxicity[J]. Revista iberoamericana de micología, 2009, 26(4): 223-227.
- Related articles
- Related Qustion
- Amphotericin B: Mechanism of Action Mar 10, 2025
Despite nearly 20 years of clinical use, the pharmacokinetics and pharmacodynamics of Liposomal Amphotericin B, which differ considerably from DAmB, remain relatively poorly understood and underutilized in the clinical setting.
- What is amphotericin B mainly used for? Mar 16, 2024
Amphotericin B is a broad-spectrum polyene antimycotic with activity against pathogenic yeasts, moulds, dimorphic fungi, and in vitro also against dermatophytes.
- Evaluation of the efficacy safety and toxicity of Amphotericin B Jan 22, 2024
Amphotericin B (AmB) is the oldest and for decades the only available agent for the treatment of life-threatening invasive fungal diseases, known for its broad-spectrum fungicidal activity against a wide range of yeasts and moulds.
RAD140 is an investigational selective androgen receptor modulator (SARM) that is developed by Radius Health, Inc.....
Aug 8,2022API3-Bromobiphenyl is an organic synthetic intermediate, which can be used as a raw material for pesticide fungicide and used as an industrial disinfectant.....
Aug 9,2022APIAmphotericin B
1397-89-3You may like
- Amphotericin B
-
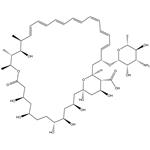
- 2025-12-13
- CAS:1397-89-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Amphotericin B
-

- $0.00 / 1g
- 2025-12-13
- CAS:1397-89-3
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1kg/month
- Amphotericin B
-
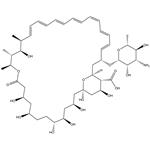
- $0.00 / 1kg
- 2025-12-13
- CAS:1397-89-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise