Naltrexone hydrochloride: impact on Cytochrome P450 and application research
May 7,2025
Introduction
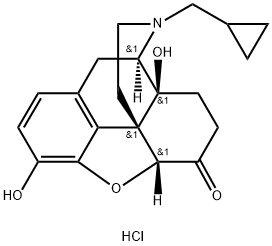
Naltrexone hydrochloride (Figure 1) is an opioid antagonist that was approved by the US Food and Drug Administration (FDA) for the treatment of heroin addiction in 1984 and alcohol dependence in 1995. For the past 2 decades, medical practitioners have believed that low-dose naltrexone hydrochloride (≤5 mg/day) could have valuable therapeutic actions on autoimmune diseases such as multiple sclerosis and inflammatory bowel disease, chronic pain, and fibromyalgia. Following oral administration, naltrexone hydrochlorideis rapidly and almost completely absorbed, and approximately, 96% of the dose is absorbed from the gastrointestinal tract. Although naltrexone hydrochloride is well absorbed orally, it is subject to extensive hepatic first-pass metabolism and, therefore, has an oral bioavailability ranging from 5 to 40%. The effectiveness of naltrexone hydrochloride is believed to be mediated by both naltrexone hydrochloride, and its 6β-naltrexol metabolite. The elimination half-life of naltrexone hydrochloride and its metabolite is 4 and 13 h, respectively, and they achieved their peak plasma levels within 1 h of dosing.[1]

1. Effect of Naltrexone Hydrochloride on Cytochrome P450 1A2,2C9, 2D6, and 3A4 Activity in Human Liver Microsomes
Cytochrome P450 (CYP) 1A2,2C9, 2D6, and 3A4 are the most important phase I drug-metabolizing enzymes in the liver, but there is a dearth of literature available on the effects of naltrexone hydrochloride on these major enzymes present in the human liver. Thus, in the present study, the effect of naltrexone hydrochloride on the activity of CYP1A2, 2C9,2D6, and 3A4 using human liver microsomes (HLM) was investigated. A selective probe for CYP1A2, 2C9, 2D6, and3A4 was incubated with HLM with or without naltrexone hydrochloride. Phenacetin O-deethylation, tolbutamide 4-hydroxylation, dextromethorphan O-demethylation, and testosterone 6β-hydroxylation reactions were monitored for enzyme activity. The activity of all the studied CYP enzymes except 1A2 was significantly inhibited by naltrexone hydrochloride 1μM. Furthermore, 1μM naltrexone hydrochloride inhibited CYP3A4 enzyme activity, the most by 37.9% followed by CYP2C9 (36.5%) and CYP2D6(31.8%). The CYP2C9 and CYP2D6 metabolic activities were greatly affected by naltrexone hydrochloride, which even at the lowest concentration of naltrexone hydrochloride (0.01μM) significantly decreased the metabolic activity by 34.9 and 16.0%, respectively. The half maximal inhibition concentration (IC50) values for CYP2C9 and CYP2D6 inhibition were 3.40±1.78 and 5.92±1.58μM, respectively. These outcomes advocate that there is a great possibility of drug interactions resulting from the concurrent administration of naltrexone hydrochloride with actives that are metabolized by these CYP enzymes, particularly CYP2C9 and CYP2D6. Nevertheless, further clarification is needed through detailed in vivo pharmacokinetic studies.[1]
2. Physicochemical Stability of Compounded Naltrexone Hydrochloride Solutions in PCCA Base SuspendIt
The purpose of this study was to determine the physicochemical stability of extemporaneously compounded naltrexone hydrochloride solutions in PCCA base SuspendIt. This base is a sugar-free, paraben-free, dye-free, and gluten-free thixotropic vehicle containing a natural sweetener obtained from the monk fruit. The study design included two naltrexone hydrochloride concentrations to provide stability documentation over a bracketed concentration range for eventual use by compounding pharmacists. A robust stability-indicating HPLC assay for the determination of the chemical stability of naltrexone hydrochloride in SuspendIt was developed and validated. Solutions of naltrexone hydrochloride were prepared in SuspendIt at 0.5-mg/mL and 5.0-mg/mL concentrations, selected to represent a range within which the drug is commonly dosed. Samples were stored in plastic, amber prescription bottles at two temperature conditions (5°C and 25°C). Samples were assayed initially, and at the following time points: 7 days, 14 days, 29 days, 44 days, 61 days, 90 days, 120 days, and 180 days. Physical data such as pH, viscosity, and appearance were also noted. All measurements were obtained in triplicate. A stable extemporaneous preparation is defined as one that retains at least 90% of the initial drug concentration throughout the sampling period. The study showed that naltrexone hydrochloride concentrations did not go below 94% of the label claim (initial drug concentration) at both temperatures studied. Viscosity and pH values also did not change significantly. This study demonstrates that naltrexone hydrochloride is physically and chemically stable in SuspendIt for 180 days in the refrigerator and at room temperature, thus providing a viable, compounded alternative for naltrexone hydrochloride in a liquid dosage form, with an extended beyond-use date to meet patient needs.[2]
3. Naltrexone Hydrochloride Use in the Treatment of Alcoholism
The development of naltrexone hydrochloride as a pharmacologic adjunct to the treatment of alcoholism was based initially on studies using animals that looked at excessive alcohol consumption. It was found that alcohol-preferring strains of mice and rats have increased basal β-endorphin levels in the pituitary gland and insome brain areas relative to alcohol-nonpreferring rats.When alcohol-preferring rats were given naltrexone hydrochloride, a pure opioid antagonist, alcohol consumption decreased. In humans, nonalcoholic persons with a strong family history of alcoholism (high risk) were compared with nonalcoholic persons with no family history of alcoholism (low risk). Baseline plasma,β-endorphinlevels were lower in the high-risk group, and a small dose of alcohol caused a substantially greater increase in plasma β-endorphin levels than in those of the low-risk group. Based on these and other studies, the endogenous opioid hypothesis was formulated that proposes that the ingestion of alcohol stimulates the release of endogenous opioids that increase some of the rewarding effects of alcohol. This is not to say that endorphins are the only neurotransmitter involved in the behavior associated with alcohol consumption; serotonin and dopamine have also been implicated, and clearly there are multiple mechanisms that need further study.[3]
Naltrexone use seems to reduce the risk of relapse but does not prevent a person with alcoholism from picking up the first drink. Thus, naltrexone may be most effective in patients with a higher risk for relapse-that is, patients with greater somatic symptoms and higher levels of alcohol craving, when accompanied by some form. of behavioral or social therapy.
References
[1] AlRabiah H, Ahad A, Mostafa GAE, Al-Jenoobi FI. Effect of Naltrexone Hydrochloride on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 Activity in Human Liver Microsomes. Eur J Drug Metab Pharmacokinet. 2018;43(6):707-713. doi:10.1007/s13318-018-0482-x
[2] Pramar YV, Mandal TK, Bostanian LA, Nguyen AT, Morris TC, Graves RA. Physicochemical Stability of Compounded Naltrexone Hydrochloride Solutions in PCCA Base SuspendIt. Int J Pharm Compd. 2019;23(2):157-162.
[3] Ey SR. Naltrexone hydrochloride use in the treatment of alcoholism. West J Med. 1996;165(4):227.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringBOC-L-Leucine is an essential amino acid for protein synthesis and can regulate various cellular processes and there are different synthesis methods.....
May 7,2025Chemical MaterialsNaltrexone hydrochloride
16676-29-2You may like
Naltrexone hydrochloride manufacturers
- Naltrexone hydrochloride
-
- 2025-12-12
- CAS:16676-29-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Naltrexone hydrochloride
-

- $0.00 / 1KG
- 2025-11-04
- CAS:16676-29-2
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 1000KG
- Naltrexone hydrochloride
-
- $1.10 / 1g
- 2025-06-25
- CAS:16676-29-2
- Min. Order: 1g
- Purity: 99.0% min
- Supply Ability: 100 tons min