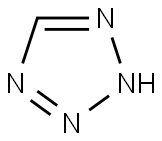
Tetrazole: Novel Building Blocks for Drug
May 7,2025
Typically, tetrazoles are introduced from their nitrile precursors through late-stage functionalization. In this work, we propose a novel strategy involving the use of diversely protected, unprecedented tetrazole aldehydes as building blocks. This approach facilitates the incorporation of the tetrazole group into multicomponent reactions or other chemistries, aiding in the creation of a variety of complex, drug-like molecules.

Synthesis of drug-like molecules using tetrazole as core building blocks
Tetrazole is considered as a privileged scaffold in pharmaceutical and medicinal chemistry, used as a carboxylic acid bioisostere and a cis-amide mimic contributing to improvements in lipophilicity, metabolic stability, conformational rigidity, and potency. Recently, the use of the tetrazole moiety in drug development has been increased and exhibited prevalent occurrence in bioactive compounds; being present in more than 20 marketed drugs with a very broad range of biological activities such as anticancer, antitubercular, antibacterial, antiviral, antimalarial, antiallergic, and antihypertensive. In addition, tetrazoles constitute a diverse range of industrial applications and are extensively used in materials, agriculture, explosives and photography. In order to achieve the synthesis of novel tetrazole building blocks and their use in organic synthesis we envisioned the use of multicomponent reactions in both steps. The use of MCRs provides the benefits of simplicity, speed, complexity, and diversity with the minimum number of steps and with an environmentally friendly nature. First, we focused on the use of the Passerini-tetrazole reaction for the synthesis of it building blocks which provides the handle of alcohol functionality and further oxidation serves as an oxo component in subsequent MCRs. The synthesis of oxo-tetrazoles was targeted because of the prevalence of the aldehyde substrate in MCRs and their use in medicinal chemistry literature.[1]
First, we planned to provide a number of orthogonally protected tetrazole carbaldehyde building blocks. This should be accomplished by synthesizing the hydroxymethyl precursors by a Passerini-tetrazole synthesis, followed by oxidation to the aldehyde. Based on our recently reported highly improved α-hydroxylmethyltetrazole synthesis under mild and save conditions we started to explore the utility of the Passerini-tetrazole reaction with cost-efficient and readily available paraformaldehyde (powder form). However, paraformaldehyde can sometimes be a challenging substrate for MCRs and this was also the case for the synthesis of oxo-tetrazoles via a Passerini tetrazole reaction. In a model reaction, we investigated benzyl isocyanide (1 equiv), paraformaldehyde (2 equiv) and trimethylsilyl azide (1 equiv) as easily available substrates. Trimethylsilyl azide is considered as a safe replacement of metal azides. We started the solvent optimization with MeOH and H2O as solvent system at room temperature, however, it did not yield any product even after 3 days. The use of DMF to improve the solubility of the paraformaldehyde solid was also unsuccessful to increase the product yield. The increased use of microwave conditions in organic synthesis and our previous promising studies on microwave-assisted MCRs, motivated us to use microwave conditions for further optimization.
Our study presented herein comprise a significant advancement in the field of medicinal chemistry and drug development. The introduction of 1H-tetrazole as a bioisostere for carboxylic acid has long been recognized for its potential in enhancing drug-like properties. Predominantly, tetrazoles are currently introduced by a late-stage-functionalization approach from their nitrile precursors. This work, however, takes an additional step forward by employing novel 1H-protected tetrazole aldehydes as versatile building blocks, a strategy not previously explored. A key innovation of this research lies in the synthesis of these tetrazole building blocks. The use of the Passerini three-component reaction (PT-3CR) utilizing cost-effective and readily available materials not only simplifies the process but also opens up avenues for gram-scale production. This method stands out for its efficiency, simplicity, and environmental friendliness, in contrast to previous syntheses. The incorporation of these tetrazole building blocks into various multicomponent reactions (MCRs) is an additional aspect of our work. This approach significantly expands the chemical space available for drug discovery, offering a means to create complex, drug-like molecules with high skeletal diversity. The study demonstrates that these tetrazole building blocks can be effectively integrated into both Passerini and Ugi reactions, indicating their broad applicability in synthesizing a wide range of molecular scaffolds.
Tetrazolium Compounds: Applications in Medicine
Tetrazoles represent a class of five-membered heterocyclic compounds with polynitrogen electron-rich planar structural features. This special structure makes tetrazole derivatives useful drugs, explosives, and other functional materials with a wide range of applications in many fields of medicine, agriculture, material science, etc. Based on our research works on azoles and other references in recent years, this review covers reported work on the synthesis and biological activities of tetrazole derivatives. Like other azole compounds, due to the relatively late start of the synthesis and study of tetrazole compounds, they did not attracted much attention in the beginning. Since 1885, when Bladin first synthesized tetrazole derivatives (2-cyanophoric-5-phenyltetrazole) to 1950, only some 300 kinds of derivatives were reported. Since the 1950s, when tetrazole compounds became widely used in agriculture, biochemistry, medicine, pharmacology, explosives and other aspects, research began to develop rapidly.[2]
The tetrazolyl functional group which was often considered as a carboxylic acid replacement in drugs, not only because the pKa is close, but it also has approximately the same planar delocalized system space requirements, and it provided a maximum nitrogen content of any heterocyclic compound. The planar ring skeleton structure and the nitrogen-rich multi-electron conjugated system confer tetrazole derivatives with both donor and acceptor electronic properties. Tetrazole and its derivatives have this attracted the interest of scientists because of their unique structures and their potential applications as antihypertensive, anti-allergic, antibiotic and anticonvulsant agents. In the present review, emphasis was focused on the diverse pharmacological properties associated with substituted tetrazoles in the past few years and a conclusive discussion on structure-activity relationship (SAR) of these compounds is provided.
A privileged scaffold for the discovery of anticancer agents
Carcinoma, characterized by abnormal growth of cells and tissue, is a ubiquitously leading cause of mortality across the globe due to some carcinogenic factors. Currently, several anticancer agents are commercially available in the global market. However, due to their resistance and cost, researchers are gaining more interest in developing newer novel potential anticancer agents. In the search for new drugs for clinical use, the tetrazole ring system has emerged as an exciting prospect in the optimization studies of promising lead molecules. Among the various heterocyclic agents, tetrazole-containing compounds have shown significant promise in the treatment of a wide range of diseases, particularly cancer. Here, in this review, we focused on several synthetic approaches for the synthesis of tetrazole analogs, their targets for treating cancer along with the biological activity of some of the recently reported tetrazole-containing anticancer agents.[3]
Over the last decade, research has focused on developing novel anticancer medications that can overcome the side effects of existing medications and be used to treat cancer patients with drug resistance. Tetrazoles have been identified as one of the most promising pharmacophores for the development of novel effective anticancer agents. Several studies have shown that tetrazoles-containing compounds can be synthesized conventionally or via green synthetic approaches. The most common method is to react nitrile with sodium azide in the presence of reagents such as ZnBr2, NH4Cl, and others. However, it can also be synthesized using a variety of other starting reagents such as aldehydes, amines or amides, aldoximes, and so on. These analogs have exhibit anticancer activity against a variety of pathways, including inhibition of PRMT1 (protein arginine methyltransferase 1) and PAD, downregulation of Ki-67 expression, and inhibition of efflux pump and tubulin formation. This review focuses on tetrazole synthetic strategies and inhibitory activities against various drug targets to provide insight into the rational design of high-efficiency tetrazole-based anticancer drug candidates.
References
[1]Li J, Chandgude AL, Zheng Q, Dömling A. Innovative synthesis of drug-like molecules using tetrazole as core building blocks. Beilstein J Org Chem. 2024 Apr 29;20:950-958.
[2]Wei CX, Bian M, Gong GH. Tetrazolium compounds: synthesis and applications in medicine. Molecules. 2015 Mar 27;20(4):5528-53.
[3]Verma, Anil et al. “Tetrazole: A privileged scaffold for the discovery of anticancer agents.” Chemical biology & drug design vol. 100,3 (2022): 419-442.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringNaltrexone hydrochloride is an orally active narcotic antagonist used to facilitate rapid transition from methadone maintenance.....
May 7,2025Drugs