PIPES: Preparation Method, Catalytic Application and Differences with HEPES
Mar 21,2024
General Description
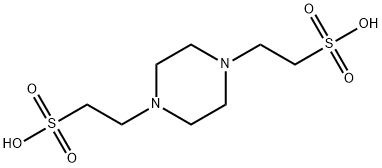
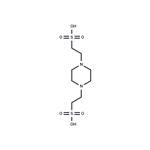
Preparation method for 1,4-piperazinediethanesulfonic acid (PIPES) involves refluxing piperazine with methanol, copper powder, and dichloroethane, followed by subsequent reactions and filtration to obtain PIPES. PIPES is highly efficient as a catalyst in the synthesis of pyrano[4,3-b]pyrans, enabling solvent-free reactions at room temperature with high yields and short reaction times. Additionally, PIPES is recyclable, enhancing its economic viability. Contrasting with HEPES, PIPES is insoluble in water and ideal for metal ion-containing buffer solutions, especially in protein purification processes. The selection of the appropriate buffer like HEPES or PIPES depends on specific experimental needs.

Figure 1. PIPES
Preparation Method
The preparation method for PIPES involves several steps as outlined below: To start, piperazine, methanol, copper powder, and dichloroethane are combined in a three-necked flask and heated to reflux. Next, a sodium hydroxide-methanol solution is slowly added, and the mixture is allowed to react for 4 hours. Water is then added, and the solution is heated to 95°C. Sodium sulfite and additional water are introduced, and the reaction is maintained for 6 hours. The resulting mixture is filtered to obtain a first filtrate, which is then cooled to room temperature. Subsequently, concentrated hydrochloric acid is added to the filtrate to adjust the pH to 1-2, leading to the formation of a first filter cake. Pure water and sodium hydroxide are added to dissolve the cake, followed by filtration to obtain a second filtrate. The pH of this solution is adjusted to 2-3 by adding concentrated hydrochloric acid and stirring for 10 minutes. The mixture is then filtered again to yield a second filter cake, which is dried at 70-80°C. This comprehensive process results in the successful preparation of PIPES, a compound crucial in various scientific and industrial applications. 1
Catalytic Application
The catalytic application of PIPES in the synthesis of pyrano[4,3-b]pyrans is demonstrated in a one-pot multicomponent reaction. This process involves the combination of benzaldehydes, malononitrile, and 4-hydroxy-6-methyl-2-pyrone using a ball milling process. PIPES exhibits high catalytic efficiency in this reaction, resulting in the formation of pyrano[4,3-b]pyrans with various substituents (e.g., Ph, 4-MeC6H4, 4-FC6H4). The use of PIPES as a catalyst in this protocol offers several advantages. Firstly, the reaction can be carried out under solvent-free conditions at room temperature, which reduces environmental impact and simplifies the procedure. Additionally, the yields obtained are high, and the reaction times are short, allowing for efficient and rapid synthesis. Furthermore, PIPES demonstrates recyclability, highlighting its potential as an economically viable catalyst. Overall, the catalytic application of PIPES in the one-pot multicomponent synthesis of pyrano[4,3-b]pyrans provides a convenient, environmentally friendly, and efficient method for the preparation of these compounds with diverse substituents. 2
Differences with HEPES
HEPES and PIPES are both types of buffer solutions, each with distinct characteristics and applications in various fields. HEPES, a zwitterionic buffer, is highly water-soluble and commonly used in cell culture and diagnostic kits. Its ability to maintain pH stability in biochemical reactions makes it a preferred choice when paired with fruit acids in cosmetics such as lotions, gels, and serums for exfoliation and skin brightening. On the other hand, PIPES is insoluble in water but soluble in aqueous sodium hydroxide solutions. It does not form stable complexes with metal ions, making it suitable for buffering solutions containing metal ions. PIPES is extensively utilized in protein purification techniques like chromatography and gel filtration, particularly in purifying recombinant GTP and binding proteins ARF1 and ARF2 from E. coli. However, PIPES is not ideal for redox systems. In terms of solubility, HEPES exhibits strong water solubility with a pH buffering range from neutral to alkaline, while PIPES is insoluble in water and has a different buffering range. Structurally, HEPES contains one -SO3H and -H group, whereas PIPES has two -SO3H groups. In conclusion, while both HEPES and PIPES serve as buffers, their differences in properties and applications highlight the importance of selecting the appropriate buffer based on the specific experimental requirements. 3
Reference
1. Xiao GG, Xu N, Liu X, Xiao GF. Method for preparation of 1,4-piperazinediethanesulfonic acid. 2021. Patent Number: CN112174912.
2. Nader GK, Mohd RJ, Taraneh M. Catalytic Application of 1,4-Piperazinediethanesulfonic Acid (PIPES) for the One-pot Multicomponent Synthesis of Pyrano[4,3-b]pyrans. Organic Preparations and Procedures International. 2020; 52(4): 368-373.
3. Roig T. Ionization enthalpies of some common zwitterionic hydrogen-ion buffers (HEPES, PIPES, HEPPS and BES) for biological research. Acta chemica Scandinavica. 1993; 472(1): 899-901.
- Related articles
- Related Qustion
- PIPES: properties, applications and safety Nov 10, 2023
PIPES is a versatile buffering agent for maintaining pH stability, widely used in biological and chemical research.
- What is 1,4-Piperazinediethanesulfonic acid (PIPES)? Feb 14, 2020
Pipes is an important chemical intermedia in all kinds of chemical fields. And 1,4-Piperazinediethanesulfonic acid, piperazine-N, N’-bis- (2-ethanesulfonic acid) (PIPES) is also one of the zwitterion buffers and the most appropriate buffer
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringMogroside V targets miR-21-5p/SPRY1 to mitigate lung inflammation by modulating pro-inflammatory factors, offering a promising anti-inflammatory therapeutic approach.....
Mar 21,2024API