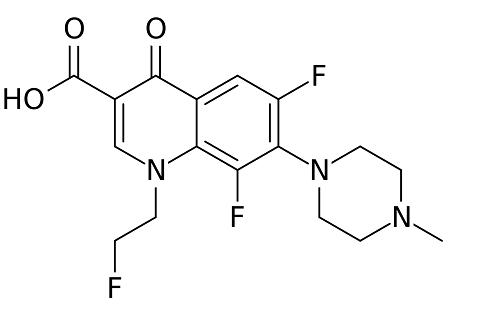
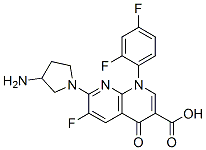
Side effects of Tosufloxacin
Mar 25,2022
Tosufloxacin was developed by Taisho-Toyama Chemistry (Ozexs) and became commercially available in 1990. It is a newer-generation oral fluoroquinolone with a broad spectrum of activity against Gramnegative, Gram-positive, and anaerobic organisms, and Chlamydophila spp. Because of concerns regarding potential toxicity, tosufloxacin is not available in the USA, but it is available in Japan and some other Asian countries, where it is used with high success rates predominantly for treatment of respiratory and gastrointestinal tract infections, as well as genitourinary, hepatobiliary, and orthopedic infections.
Uses
Tosufloxacin has been used with high success rates for a broad variety of conditions. Recent clinical data about its effectiveness are limited mainly to Japanese-language journals, but in those countries where it is still available, it may be considered for the following indications.
Bioavailability
Bioavailability is high, but is reduced with co-administration of aluminum hydroxide or preparations of iron, calcium, or magnesium.
Excretion
Peak urinary concentrations are obtained approximately 2 hours after dosing with levels of 64.1 and 115.8 mg/ml measured after doses of 150 and 300 mg, respectively. Urinary recovery rates of 39% and 46% were seen at 12 and 24 hours, respectively, following the 150-mg dose.
Side effects
Adverse reactions are not common and consist mainly of gastrointestinal upset, rashes (including photosensitivity), headaches, and minor abnormalities of liver function tests. The most significant adverse event is rhabdomyolysis, although this is not common. There has not been significant association with tendonopathy, QT interval prolongation, hypoglycemia, or liver failure.
- Related articles
- Related Qustion
In the 1970s, Beecham Research Laboratories identified a carbapenem group, called olivanic acids, which were beta-lactamase inhibitors and broad-spectrum antibiotics (Butterworth et al., 1979).....
Mar 25,2022APIFleroxacin (Ro 23-6240, AM 833) is different from fluoroquinolones such as ciprofloxacin or ofloxacin, since it is a trifluorinated quinolone, having three fluorine atoms rather than one attached to the quinolone ring system. It is a 6,8-di....
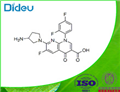
Mar 25,2022APITosufloxacin
108138-46-1You may like
- Tosufloxacin
-
- $80.00 / 1mg
- 2025-12-10
- CAS:100490-36-6
- Min. Order:
- Purity: 99.48%
- Supply Ability: 10g
- Tosufloxacin USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:108138-46-1
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min