Total synthesis of colchicine
Dec 9,2025
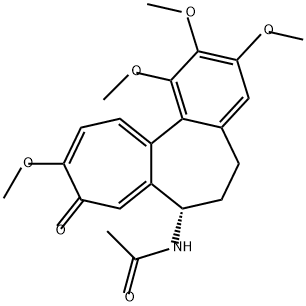
Colchicine has been isolated from, inter uliu, the meadow saffron Colchicum autumnale and might well be described as the prototypic anti-mitotic drug although clinical applications of this alkaloid are restricted because of its toxicity.
Banwell 1996 total synthesis of colchicine
The useful biological properties and novel structure of this compound has resulted in considerable effort being directed towards its synthesis. It has been suggested that the troponoid C-ring of colchicine is formed late in the biosynthetic process, possibly by the route illustrated in Scheme 1. This suggestion prompted us to examine whether elements of such a pathway could be mimicked in the laboratory thus permitting development of what would be the first regio- and enantio- controlled total synthesis of (1). Such an approach has been very fruitful.
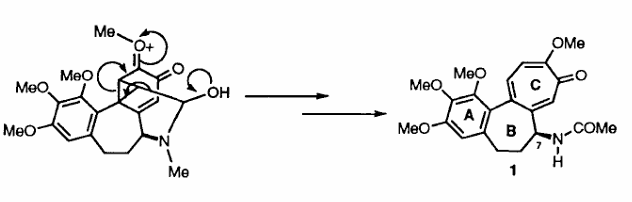
Scheme 1: Late Stages in the Biosynthesis of Colchicine (1)
Our synthesis (Scheme 2) begins with benzaldehyde (2) and acetophenone (3) which are readily elaborated, via an initial Claisen-Schmidt condensation reaction, to the 1.3-diarylpropanol (4). Subjection of this last compound to an oxidative coupling protocol developed by Umezawa7 ultimately afforded the dibenzocycloheptenone (5). In anticipation of the enantioselective introduction of the C-7 acetamido group associated with (1) it was necessary, for various reasons, to form alcohol (6) with the R- configuration at C-7 (colchicine numbering). To these ends, the ketone (5) was subjected to enantioselective reduction with stoichiometric quantities of the CBS-reagent8 and, after debenzylation, the target compound (6) was obtained in 94% ee. Taylor-McKillop oxidation9 of this phenol followed by nucleophilic c yclopropanation of the derived cyclohexadienone (7) with dimethylsulfoxonium methylide then provided the key a-homo-o-benzoquinone mono-acetal(8) (98% ee after one recrystallisation), the structure (including absolute configuration) of which was established by single-crystal X-ray analysis.[1]
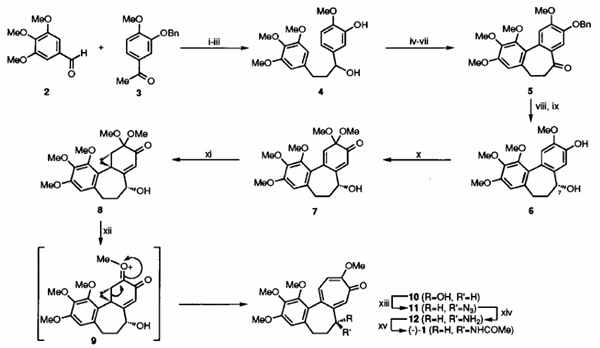
Scheme 2: Total Synthesis of (-)-Colchicine (1)
Reagents and conditions: (i) NaOH, MeOH, RT, 48h, 96%; (ii) H2, Pd on C, MeC02Et, 15OC, 10h, 96%; (iii) NaBb, W/MeOH, 15"C, lSh, 96%; (iv) Pb(OCOMe)q, 3A sieves, CH2C12, 15°C lh, 100%; (v) CF~COZH, 3A sieves, THF/C&j, O'C, lh, 42%; (vi) BnBr, K2CO3, MeCN, 82"C, 4h, 88%; (vii) NMO, "PAP, 4A sieves, CH2C12, 15OC, 43h, 98%; (viii) CBS-reagent, THF, 15OC. 6h, 88%; (ix) H2. Pd on C. MeC@Et, 15OC, 9h, 99%; (x) m(No3)3, MeOH, -2OoC, OSh, 83%; (xi) Me3S(O)I, NaH, DMSO, 15'C, 7h, 54% @ 82% conversion; (xii) CF3CO2H. CH2C12, 15OC, 3h, 48%; (xiii) i-Pr02CN=NC02Pr-i, PPh3, Zn(N3)2-2(CgHgN), THF, 15"C, 38h, 30%; (xiv) PPh3. H20, THF, 15OC, 63h; (xv) (MeC0)20, CgHgN, 15°C. 0.25h, 60% from 11.
Reaction of compound (8) with trifluoroacetic acid in dichloromethane resulted in the desired (biomimetic) ring-expansion and formation of troponoid (10) (98% ee). Most likely, this key conversion proceeds via the oxonium ion (9). Mitsunobu chemistryll was used to effect sN2 displacement of the hydroxy group in compound (10) by azide ion. The resulting azido-compound (11) (> 95% ee) was subjected to reduction under Staudinger conditions and the amine (12) so-formed was immediately acetylated thereby affording colchicine (1) (> 81%ee).
References
[1] Banwell, M. “Cyclopropyl compounds as chemical building blocks: Total syntheses of the alkaloids (-)-colchicine, imerubrine and grandirubrine.” Pure and Applied Chemistry 68 1 (1996): 539–542.
- Related articles
- Related Qustion
- Colchicine: A Versatile Anti-Inflammatory Medication for Cardiovascular Disease Jan 9, 2024
Colchicine has shown clinical efficacy in the treatment of pericardial disease and atrial fibrillation, with common side effects including gastrointestinal intolerance and myalgias.
- Colchicine: mechanism of action, activities and side effects Jul 24, 2023
Colchicine binds to tubulins, disrupting microtubule assembly. It has anti-inflammatory, anti-fibrotic, and cardiovascular protective effects but can cause various side effects.
- Uses of Colchicine Jan 11, 2022
Colchicine is obtained from the autumn crocus, Colchicum autumnale, or the glory lily, Gloriosa superba.Descriptions regarding use of Colchicum autumnale (meadow saffron) for swelling and types of inflammatory arthritis date back to the fir
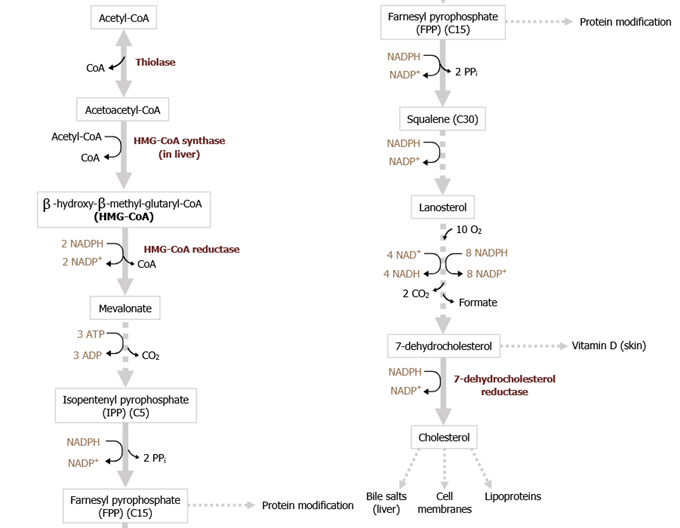
Cholesterol is either synthesised de novo in our cells or is taken in the form of dietary cholesterol from food sources. This article will introduce its synthesis pathway in human body.....
Dec 9,2025Biochemical EngineeringColchicine
64-86-8You may like
- Colchicine
-
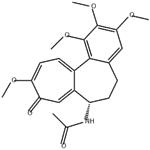
- 2025-12-11
- CAS:64-86-8
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Colchicine
-

- $0.00 / 1KG
- 2025-12-11
- CAS:64-86-8
- Min. Order: 1KG
- Purity: ≥98% HPLC
- Supply Ability: 1000KG
- Colchicine
-

- $1.00 / 1kg
- 2025-12-11
- CAS:64-86-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt