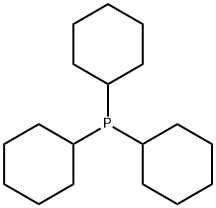
Synthesis method of tricyclohexyl phosphine
Aug 18,2022
Introduction
Tricyclohexyl phosphine is the tertiary phosphine with the formula P ( C 6 H 11) 3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to PCy 3, where Cy stands for cyclohexyl. It is characterized by both high basicity (p K a = 9.7) and a large ligand cone angle (170°). Tricyclohexyl-phosphine is more basic (and so a better c-donor) than tribu tyiphosphine, which in turn is more basic than triphenyl phosphIne[1].
Synthesis method of tricyclohexyl phosphine
The invention relates to a synthesis method of tricyclohexyl phosphine[2]. The method adopts a one-pot process and comprises the following steps: carrying out Grignard reaction on chlorocyclohexane and magnesium powder used as raw materials to obtain a cyclohexyl Grignard reagent, and carrying out condensation reaction on the cyclohexyl Grignard reagent and phosphorus trichloride; and after the reaction finishes, slowly and dropwisely adding carbon bisulfide, refluxing to generate complexing reaction, carrying out vacuum filtration, washing and drying to obtain the stable tricyclohexyl phosphine carbon bisulfide complex. The method solves the problem of low stability and high oxidation tendency of the tricyclohexyl, has the advantages of accessible reaction raw materials, simple synthesis process, low cost, simple operation of after-treatment, small environmental pollution, high reaction operation safety, high reaction yield and favorable product quality, and is beneficial to industrialization.
Preparation method of tricyclohexyl phosphine
The invention relates to a preparation method of tricyclohexyl phosphine, which comprises the following steps: reacting a cyclohexyl Grignard reagent and phosphorus trihalide[3], dropwisely adding a saturated strongly-acidic weakly-alkaline inorganic salt water solution at low temperature to carry out quenching, adding tetrafluoroboric acid to prepare a salt, and resolving the salt with triethylamine to obtain the tricyclohexyl phosphine, wherein the cyclohexyl Grignard reagent is prepared by carrying out Grignard reaction on halogenated cyclohexane and magnesium metal under inert gas shielding. According to the invention, the product is subjected to after-treatment by a chemical process; the tetrafluoroboric acid firstly reacts with tricyclohexyl phosphine to form a salt to completely extract the tricyclohexyl phosphine from the reaction solution, and the salt is resolved with the triethylamine. The invention has very obvious beneficial effects, and greatly enhances the preparation yield; and the prepared product has the advantages of high purity, high quality and low preparation cost.
Application in olefin metathesis reactions
The use of tricyclohexylphosphine as a ligand in Grubbs catalysts has greatly advanced the development of olefin metathesis reactions[4]. The first generation of Grubbs catalyst was produced in 1995, which not only has strong resistance to oxygen and water, but also has good functional group compatibility, and the catalytic activity is also greatly improved. In 1999, Grubbs et al. used a saturated nitrogen heterocyclic carbene to replace one of the PCy3 catalysts to obtain a second-generation catalyst, which not only maintained the advantages of the first-generation catalyst, but also improved its thermal stability and catalytic activity. In the ring-closing metathesis reaction, only 0.05 mol% of this catalyst is used. In ring-opening metathesis polymerization, only 0.0001 mol% is required[5].
Application in crabtree catalysts
Tricyclohexyl phosphine can be used as a ligand in Crabtree catalysts. Crabtree catalyst is a very convenient ionic ligand. The catalyst is used as a homogeneous reduction catalyst, which is superior to Wilkinson's catalyst, and can also achieve homogeneous catalytic reduction of tetra-substituted olefins, which are difficult to reduce. At the same time, if the substrate contains a directing group, a directed selective reduction can also be performed[6].
Tricyclohexyl phosphine can also be used as a ligand to synthesize palladium catalyst for efficient Suzuki cross-coupling reaction of chlorinated aromatic hydrocarbons[7]. There is an unshared electron pair on the central phosphorus atom of tricyclohexylphosphine, which can also be used as an initiator. As a neutral nucleophile, it initiates the polymerization of acrylonitrile. During initiation and growth, charge-separated zwitterions are generated. Due to its weak activity, the polymerization of active monomers can be initiated under milder conditions.

Picture 1 Application of Tricyclohexyl phosphine[8]
Reference
1 Grubbs, R. H.; Vougioukalakis, G. C. Chem. Rev. 2010, 110,1746.
2 Synthesis method of tricyclohexyl phosphine, CN106749399A ? 2017-05-31 ? WEI CHENGGONG
3 Preparation method of tricyclohexyl phosphine CN102875596A (B) ? 2013-01-16 ? BEIJING GREENCHEM TECHNOLOGY CO LTD
4 Crabtree, R. H. Acc. Chem. Res. 1979, 12, 331.
5 Scholl, M.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953.
6 Gong, J. F.; Liu, G. Y.; Du, C. X.; et al. J. Org. Chem.2005, 690, 3963.
7 Sehaper, F.; Foley, S. R.; Jordan, R. F. J. Am. Chem. Soc.2004, 126, 2114.
8 Jin, H., Lai, J., & Huang, Y. (2019). Phosphine-Catalyzed Domino [3 + 3] Cyclization of para-Quinamines with Morita–Baylis–Hillman Carbonates: Access to Hydroquinoline Derivatives. Organic Letters, 21(8), 2843–2846.
- Related articles
- Related Qustion
- Tricyclohexyl Phosphine: Applications in Catalytic Reactions and its Radical Synthesis Method Jul 15, 2024
Tricyclohexyl phosphine facilitates [3+2] cycloaddition with [60]fullerene, catalyzes Suzuki and Heck reactions, and is synthesized via radical titanium-catalyzed tetraphosphorus transformation.
Tedizolid is the first FDA-approved second-generation oxazolidinone antibiotic, which is clinically used to treat skin and skin tissue infections, and its efficacy is non-inferior to linezolid.....
Aug 17,2022APITricyclohexyl phosphine
2622-14-2You may like
Tricyclohexyl phosphine manufacturers
- Tricyclohexylphosphine
-

- $1.00 / 1KG
- 2025-12-12
- CAS:2622-14-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 tons
- Tricyclohexyl phosphine
-
- $0.00 / 25kg
- 2025-12-01
- CAS:2622-14-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- Tricyclohexyl phosphine
-
- $0.00 / 100g
- 2025-08-02
- CAS:2622-14-2
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 500kg