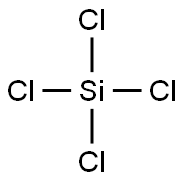
Synthesis of Silicon tetrachloride
Jan 7,2022
Silicon tetrachloride or tetrachlorosilane is the inorganic compound with the formula SiCl4. It is a colourless volatile liquid that fumes in air. It is used to produce high purity silicon and silica for commercial applications.

Synthesis
Silicon tetrachloride (SiCl4) can be manufactured by chlorination of silicon compounds such as ferrosilicon or silicon carbide, or by heating silicon dioxide and carbon in a stream of chlorine. It can also be obtained as a by-product in the production of zirconium tetrachloride, and in the past substantial quantities were produced by this route, which in recent decades has lost importance owing to the reduced demand for zirconium in nuclear facilities. Nowadays, industrial silicon tetrachloride is produced either by direct reaction of hydrogen chloride with silicon – this product mainly being employed as an intermediate in fumed silica production – or as the by-product of the production of silane for the microelectronics industry by disproportionation of trichlorosilane.
Property
Silicon tetrachloride is a colorless, noninflammable, volatile
liquid with a pungent, suffocating odor. It fumes in air and is
corrosive to metals and tissues in the presence of moisture. In
experiments at Argonne National Laboratory in which it was
mixed with water and stirred under room conditions, about
35% of the theoretical yield of HCl evolved as a gas in the first
minute. It also reacts very rapidly with alcohols, primary and
secondary amines, ammonia, and other compounds containing
active hydrogen atoms. Thermal decomposition or burning
may produce dense white clouds of silicon oxide particles and
hydrogen chloride.
Environmental Fate
Silicon tetrachloride is a by-product in the production of polysilicon, the key component of sunlight-capturing wafers in solar energy panels, and for each ton of polysilicon produced, at least four tons of silicon tetrachloride liquid waste are generated. Pollution by silicon tetrachloride has been reported in China, associated with the increased demand for photovoltaic cells that has been stimulated by subsidy programs.
Studies of rats subjected to acute inhalation of 10 structurally similar chlorosilanes, including tetrachlorosilane, suggest that the acute toxicity of chlorosilanes is largely due to the hydrogen chloride hydrolysis product. The observed effects were similar to those of HCl inhalation both qualitatively (clinical signs) and quantitatively (molar equivalents of hydrogen chloride at the atmospheric LC50).
- Related articles
- Related Qustion
- Is SiCl4 Polar or Nonpolar? Dec 27, 2023
SiCl4 is a colorless, noninflammable, volatile liquid with a pungent, suffocating odor.
Chloroquine (CQ) and hydroxychloroquine (HCQ) are synthetic 4-aminoquinolines. CQ has been used as an antimalarial drug since World War II. Later, CQ’s use expanded to include management of systemic lupus erythematosus (SLE) and rheumatoid....
Jan 7,2022APIBronopol is used as a preservative in various cosmetic, pharmaceutical, toiletry and household preparations at concentrations of up to 0.1% (wt/vol) particularly because of its high activity against Gram-negative bacteria, especially Pseudo....
Jan 7,2022Chemical ReagentsTetrachlorosilane
10026-04-7You may like
Tetrachlorosilane manufacturers
- Tetrachlorosilane
-
- $10.00 / 1KG
- 2025-12-11
- CAS:10026-04-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Tetrachlorosilane
-

- $7.98/ kg
- 2025-07-17
- CAS:10026-04-7
- Min. Order: 1kg
- Purity: 98.86%
- Supply Ability: 18000 tons per year
- Tetrachlorosilane
-
- $1.00 / 1kg
- 2019-07-06
- CAS:10026-04-7
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 500kg