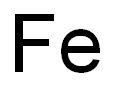The uses and isotopes of Fe
May 27,2024
Fe isotopes
The Fe isotopes include four stable ones: 54Fe (5.845% natural abundance), 56Fe (91.754%), 57Fe (2.119%), and 58Fe (0.282%). A couple dozen of isotopes have also been synthesized. Of these stable isotopes, only 57Fe has a nuclear spin (21/2). The nuclide 54Fe hypothetically can undergo double electron capture to form 54Cr. However, the process has never been detected, and only a lower limit on the half-life of 3.1 3 1022 years has been determined. 60Fe is an extinct radionuclide with a long half-life of 2.6 million years). It is not present on Earth, but its final decay product is its granddaughter, the stable nuclide 60Ni.
Uses
Iron is the most commonly used of all the metals, making up about 90% of global metal production. Its low cost and high strength frequently make it the best material to withstand stress or transmit forces, such as constructing machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings.
Steel
Pure Fe is rather soft, so it is generally combined with alloying elements to make steel. However, the mechanical properties of Fe are significantly affected by the sample's purity: pure, single crystals of Fe are softer than Al. An increase in the C concentration will substantially increase Fe's hardness and tensile strength. Maximum hardness is obtained with 0.6% C content, but the alloy has low tensile strength. Due to the softness of Fe, it is much easier to work with than its heavier congeners, ruthenium, and osmium.

α-Fe is a relatively soft metal that can dissolve only a small amount of C (<0.021% by mass at 910℃). Austenite (γ-Fe) is equally soft and metallic but can dissolve substantially more C (up to 2.04% by mass at 1146℃). This form of Fe is used in the type of stainless steel used for cutlery and hospital and food service equipment. Commercially available Fe is categorized based on purity and the abundance of additives. Pig iron has 3.5%-4.5% C and has variable quantities of contaminants, for example, S, Si, and P. Pig iron is not a marketable product but rather an intermediate material in the production of cast iron and steel. The decrease in contaminants in pig iron negatively affects material properties such as S and P, yielding cast iron containing 2%-4% C, 1%-6% Si, and trace amounts of Mn. Pig iron has a melting point of 1145 ℃-1200 ℃, which is lower than either of its two main components and makes it the first product to be melted when C and Fe are heated together. Its mechanical properties differ enormously depending on the form of the C in the alloy.
"White" cast irons have the C in the form of cementite or iron carbide (Fe3C). This hard, brittle compound dictates the mechanical properties of white cast irons, making them hard but resistant to shock. Cooling a mixture of Fe with 0.8% C slowly below 723℃ to room temperature results in separate, alternating cementite and α-Fe layers, which are soft and malleable and are known as pearlite due to their appearance. Rapid cooling, in contrast, does not allow time for this separation and results in hard and brittle martensite. The steel can subsequently be tempered by reheating to a temperature in between, changing the proportions of pearlite and martensite. The final product below 0.8% C content is a pearlite-αFe mixture, and that above 0.8% C content is a pearlite-cementite mixture. In gray iron, the C exists as separate, fine flakes of graphite, which makes the material brittle due to the sharp-edged graphite flakes that produce stress concentration sites within the material. A newer modification of gray iron, described as ductile iron, is specially treated with trace quantities of Mg to change the shape of graphite to spheroids or nodules, lowering the stress concentrations and significantly increasing the toughness and strength of the material. Wrought iron contains 0.25% C but large amounts of slag, giving it a fibrous characteristic. It is a tough, malleable material but not as fusible as pig iron. Wrought iron contains fine fibers of slag entrapped within the metal. It is more corrosion-resistant than steel. It has been almost completely superseded by mild steel for traditional "wrought iron" products and blacksmithing.
- Related articles
- Related Qustion
- What is Iron flame test Feb 2, 2024
The positive result of a flame test for iron (Fe) produces a golden color. This is due to the excitation of electrons in the sample by the flame's heat. As these electrons lose their energy, they emit photons of golden light.
- What is Iron? Aug 13, 2021
Iron is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in fro
- Iron - Drug Discovery and Legand Oct 16, 2020
Iron appears to be the first mineral known to have been administered by mouth for therapeutic purposes.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringHydrogen gas is gas is very rare in the Earth's atmosphere (1 ppm by volume) because of its lightweight, which enables it to escape from Earth's gravity more easily than heavier gases.....
May 27,2024Inorganic chemistry