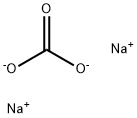
Toxicity of Sodium carbonate
Feb 7,2022

Pharmacodynamics
Alkalizing buffering action: Sodium bicarbonate is an alkalinizing agent that dissociates to provide bicarbonate ion. Bicarbonate in excess of that needed to buffer hydrogen ions causes systemic alkalinization and, when excreted, urine alkalinization as well. Oral antacid action: Taken orally, sodium bicarbonate neutralizes stomach acid by the above mechanism.
Mechanism of action
Carbon dioxide from the tissues diffuses rapidly into red blood cells, where it is hydrated with water to form carbonic acid. This reaction is accelerated by carbonic anhydrase, an enzyme present in high concentrations in red blood cells. The carbonic acid formed dissociates into bicarbonate and hydrogen ions. Most of the bicarbonate ions diffuse into the plasma. Since the ratio of H2CO3 to dissolved CO2 is constant at equilibrium, pH may be expressed in terms of bicarbonate ion concentration and partial pressure of CO2 by means of the Henderson-Hasselbach equation: pH = pk + log [HCO3-]/aPCO2.
Toxicity
Man: LD50 (Oral) - 714 mg/kg, Effect: Behavioural,General Anesthetic : GI Ulceration or Bleeding from small intestine. Mouse : LC50 ( Inhalation ) - 1200mg/m3/2h : GI Other Change Mouse : LC50 ( Intraperitoneal ) - 117mg/kg Mouse : LD50 ( Oral) - 6600mg/kg Mouse : LD50 (Subcutaneous ) - 2210 mg/kg Rat : LC50 ( Inhalation ) 2300mg/m3/2H Rat: LD50 (Oral) - 4090 mg/kg.
- Related articles
- Related Qustion
- What is soda ash used for? Mar 13, 2024
Over half of all Soda Ash production is used in glass manufacturing, but it is used in the soap industry, paper production, pH adjustment, production of cleaners and detergents, and in the polymer and resin industries.
- Soda Ash Vs Baking Soda: Similarity and Differences Mar 7, 2024
Soda Ash(Sodium carbonate), Na2CO3.10H2O, is an industrial chemical with the formula Na2CO3. Baking soda, also known as sodium bicarbonate or bicarbonate of soda, is a popular baking ingredient.
- Sodium carbonate: Applications and Security Jan 2, 2024
Sodium carbonate is the disodium carbonate salt with an alkaline base. It is widely used in industry.
Vericiguat, sold under the brand name Verquvo, is a medication used to reduce the risk of cardiovascular death and heart failure.It is taken by mouth.Common side effects include low blood pressure and low red cell count (anemia).....
Feb 7,2022APISodium Carbonate is the disodium salt of carbonic acid with alkalinizing property. When dissolved in water, sodium carbonate forms carbonic acid and sodium hydroxide. As a strong base, sodium hydroxide neutralizes gastric acid thereby actin....
Feb 7,2022Inorganic chemistrySodium carbonate
497-19-8You may like
Sodium carbonate manufacturers
- Sodium carbonate
-

- $1.00 / 1g
- 2025-12-14
- CAS:497-19-8
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- sodium carbonate
-

- $1.00 / 1kg
- 2025-12-11
- CAS:497-19-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Sodium carbonate
-

- $100.00 / 1kg
- 2025-12-10
- CAS:497-19-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000Ton