Uses and Preparation of Uracil
Jul 6,2022
General description
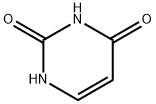
Uracil is an organic compound with molecular formula of C4H4N2O2 and molecular weight of 112.087. It is a fine gray white crystalline powder and a tautomer of a (4S)-4-hydroxy-3,4-dihydropyrimidin-2(1H)-one. Uracil is a common and naturally occurring pyrimidine nucleobase in which the pyrimidine ring is substituted with two oxo groups at positions 2 and 4. Found in RNA, it base pairs with adenine and replaces thymine during DNA transcription. It has a role as a prodrug, a human metabolite, a Daphnia magna metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and an allergen[1]. It was isolated from herring sperm and also produced in a laboratory in 1900–1901. When combined with the sugar ribose in a glycosidic linkage, uracil forms a derivative called uridine (a nucleoside), which in turn can be phosphorylated with from one to three phosphoric acid groups, yielding respectively the three nucleotides UMP (uridine monophosphate), UDP (uridine diphosphate), and UTP (uridine triphosphate).
USE
Uracil is a compound with 2,4-dihydroxypyrimidine structure, which is one of the four main bases of RNA, and has a wide range of applications in the fields of medicine, pesticide and chemical synthesis[2]. Uracil can be used as the raw material for the synthesis of many pyrimidine herbicides, such as cyclapyr, chlorpyrifos, and teripyr, which achieve herbicidal efficacy by selectively inhibiting photosynthesis[3,4]. In addition, as a special structural compound closely related to life activities, uracil plays an indispensable role in the design and application of medicine. For example, 5-fluorouracil and its derivatives synthesized from uracil have achieved good clinical effects on a variety of cancers. As a kind of drug, Uracil has the effect of anti megaloblastic anemia, treating liver, cerebrovascular, cardiovascular and other diseases. It is also the main raw material for the manufacture of fluorouracil (s-fc), deoxynucleoside, idur, BUdR, FUDR and other drugs.
Mechanism of action
The analogous nucleosides and nucleotides formed from uridine and deoxyribose occur only very rarely in living systems, such is not the case with the other pyrimidines. The nucleotide derivatives of uracil perform important functions in cellular metabolism, particularly in carbohydrate metabolism. UTP acts as a coenzyme in the biosynthesis of sucrose in plants, lactose and glycogen in mammals, and chitin in insects[5]. It can also readily donate one of its phosphate groups to adenosine diphosphate (ADP) to form adenosine triphosphate (ATP), an extremely important intermediate in the transfer of chemical energy in living cells. Since the uracil nucleotides contain only ribose and not deoxyribose, UTP is the source of uridine only in ribonucleic acid (RNA); there is no uridine in deoxyribonucleic acid (DNA). Its involvement in the biosynthesis of RNA demonstrates that uracil is important in the translation of genetic information. A few laboratory derivatives of uracil have been designed as experimental antimetabolites for use in cancer chemotherapy.
Preparation
1. Oxidative synthesis of uracil from β-dicarbonyl compounds and their analogs. Commonly used β-dicarbonyl compound derivatives include malic acid, maleic acid, propylene, propynoic acid, etc., which are dehydrated and cyclized after oxidation under the action of oleum.
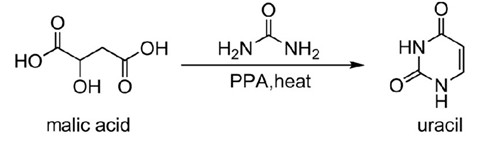
Scheme 1. Oxidative synthesis of uracil
2. Synthesis of uracil from cytosine by hydrolysis. Uracil is synthesized by hydrolyzing cytosine under acidic conditions and removing 1 molecule of ammonia gas, but because the reaction yield is low and the value of cytosine is higher than that of uracil, this reaction has no practical application value.
3. Synthesis of uracil from whey acid by photocatalytic decarboxylation. The reaction undergoes a radical process to form uracil, and adding a catalytic amount of copper ions or iron ions to the reaction can catalyze the reaction rate and improve the reaction yield.
4. Photocatalytic Dehydrogenation of β-Alanine to Synthesize Uracil. Using β-alanine as raw material and urea to deaminate under heating conditions to form β-ureidoalanine, then dehydration and condensation to form dihydrouracil, and finally photocatalytic dehydrogenation to form uracil.
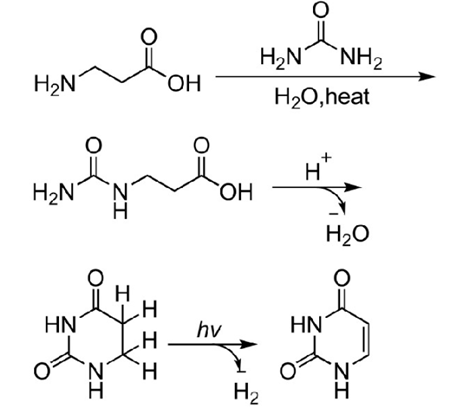
Scheme 2. Photocatalytic dehydrogenation
5. One pot synthesis of uracil from ethyl acetate and ethyl formate. Uracil was synthesized from ethyl formate and ethyl acetate in one pot at room temperature and pressure. Compared with the previous route, this route is not only cheap and easy to obtain raw materials, but also easy to operate, and the yield has been greatly improved[6]. The yield reported in the literature can reach more than 70%, which is suitable for industrial production.
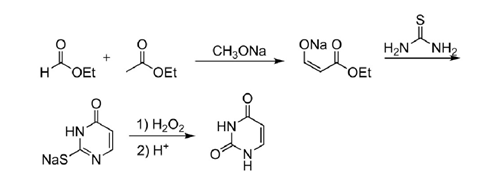
Scheme 3. One pot synthesis of uracil
References
- Merck Index - O'Neil MJ, Heckelman PE, Dobbelaar PH, Roman KJ (eds). The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th Ed. Cambridge, UK: The Royal Society of Chemistry, 2013.
- GARRETT R H, GRISHAM C M. Principals of Biochemistry with a Human Focus[M]. United States:Brooks/Cole Thomson Learning, 1997.
- YU Ai-ming, YANG Hua-zheng, LIU Hua-yin, et al. Structure Based Library Approach to Photosynthesis Inhibitors:Combinatorial Synthesis of Hydrouracil Library[J]. Science in China Series B:Chemistry, 1998, 41(5):455.
- POZHARSKII A F, KATRITZKY A R, SOLDATENKOV A.Heterocycles in Life and Society:An Introduction to Heterocyclic Chemistry and Biochemistry and the Role of Heterocycles in Science, Technology, Medicine, and Agriculture[M]. New York:John Wiley and Sons, 1997.
- Uracil.[J].Columbia Electronic Encyclopedia, 6th Edition.2019:1.
- WANG Limin WANG Siyao ZHANG Shiti YAN Dongxue JIA Yunhong. Study on the Synthesis of Uracil as a Pharmaceutical Intermediate[J]. Journal of Jinzhou Medical University, 2014,35(06):7-9.
- Related articles
- Related Qustion
- Molecular structure differences between Uracil and thymine Dec 16, 2024
Uracil (U) and thymine (T) are both nitrogenous bases in the genetic information of genes and play an important role in the structure and function of nucleic acids (DNA and RNA).
- What is the relationship between Uracil and Uracil-DNA glycosylase? Dec 16, 2024
Uracil is an important component of RNA and is closely related to the transmission of genetic traits. Uracil can be used to repair and select large-scale DNA sequence deletions.
- Uracil: Different sources in DNA and Role in Adaptive Immunity Oct 29, 2024
Uracil is one of the four nucleotide bases in the nucleic acid RNA. In RNA, uracil binds to adenine via two hydrogen bonds.
The essential trace mineral, selenium, is of fundamental importance to human health. As a constituent of selenoproteins, selenium has structural and enzymic roles, in the latter context being best-kno....
Jul 6,2022APIMonosodium glutamate is widely used to intensify and enhance umami flavors in sauces, broths, soups and many more foods.....
Jul 7,2022Food Additives