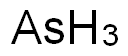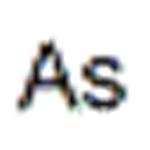Uses and safety of high-purity arsenic
Mar 16,2024
The demand for metallic arsenic is limited. It is used in nonferrous alloys, and high-purity arsenic is used in electronic and semiconductor devices. The addition of ca. 0.5 % to the lead grid in lead–acid storage batteries increases endurance and corrosion resistance.

Uses of high-purity arsenic
High-purity arsenic (at least 99.999 %) is used in electronics in conjunction with gallium or indium for the production of diodes (LED), infrared detectors, and lasers. Solar cells based on gallium arsenide have an efficiency of 20%. Among the semiconductors, silicon-based materials still dominate the market and will continue Ductions costs are far lower than those of the gallium arsenide semiconductors. Certain niches of properties that can not be provided by silicon-based semiconductors are filled by gallium arsenide, but although this market is growing, it will be limited in the future.
Safety Measures
Although pure metallic arsenic is not poisonous, the occurrence of toxic arsenic oxides formed by oxidation in the air is unavoidable. In the handling or manufacture of arsenic materials, inhalation of dust and skin contact with arsenic-containing gases must be prevented.
Acute Toxicity
Arsenic poisoning mostly occurs by ingestion of inorganic trivalent compounds such as arsenic trioxide. The smallest doses of As2O3 reported to be fatal after ingestion are 70–180mg. Symptoms that can occur within a few minutes or after a delay of several hours are vomiting, diarrhea, damage of the intestinal tract, muscular cramps, facial edema, cardiac abnormalities, dehydration, and– as a consequence– shock.
The inhalation of irritant arsenic compounds, such as arsenic trichloride or arsenical war gases, causes cough, dyspnea, pain in the chest, severe damage to the respiratory tract, headache, general weakness, nausea, vomiting, and colic diarrhea. Airborne dust often results in irritation of exposed skin and mucous membranes such as bronchitis, conjunctivitis, laryngitis, and dermatitis. Arsenic trichloride may be absorbed through the skin with fatal consequences.
Chronic Toxicity
Chronic arsenic poisoning can be caused by intake of food and water contaminated with arsenicals or by inhalation of airborne dust during long-term occupational exposure, mainly in smelting plants of ores containing arsenic, insecticide factories, and among vineyard workers, even many years after termination of exposure. Symptoms of these chronic poisonings were symmetrical palmar and plantar hyperkeratosis, white striae of the fingernails, cardiovascular manifestations, myocardial ischemia, hypertension, liver dysfunctions, hema topological changes, and vascular disorders resulting in gangrenes of the lower extremities (black foot disease).
- Related articles
- Related Qustion
- Toxicity of Arsenic Nov 16, 2021
The word arsenic has several derivations including from the Syriac word (al) zarniqa, the Persian word zarnikh,and the Greek word arsenikon (Arsεnikón). It is also related to the similar Greek word arsenikos, meaning ‘masculine’ or ‘potent.
- Arsenic——Exposure Sources & Medical Management Mar 5, 2020
Arsenic (As; CAS #7440-38-2) is a brittle solid with a metallic coloring that ranges from silver to gray. It is a naturally occurring element found in the earth’s crust,
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringBeryllium, Be, is the first element in the second main group of the periodic table. It is a light metal with a hexagonal-closest-packed (hcp) structure.....
Mar 16,2024Inorganic chemistry