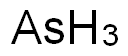Arsenic
- CAS No.
- 7440-38-2
- Chemical Name:
- Arsenic
- Synonyms
- arsane;Arsenicals;Arsenic (As);ARSENIC METAL;ARSENIC STANDARD SOLUTION;Arsen;ARSENIC;Arsenic1;Arsenium;Arsenic-75
- CBNumber:
- CB2761163
- Molecular Formula:
- AsH3
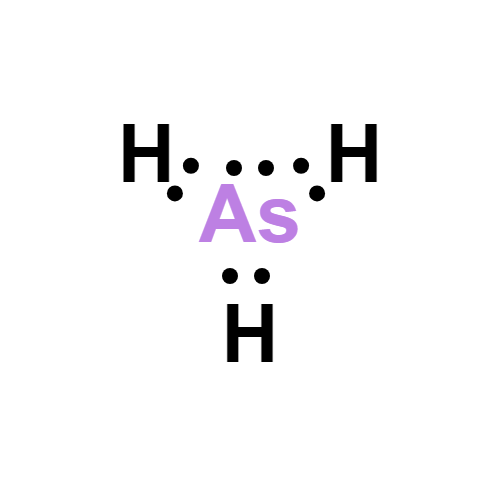
Lewis structure
- Molecular Weight:
- 77.95
- MDL Number:
- MFCD00085309
- MOL File:
- 7440-38-2.mol
- MSDS File:
- SDS
| Melting point | 817 °C(lit.) |
|---|---|
| Boiling point | 613 °C(lit.) |
| Density | 5.727 g/mL at 25 °C(lit.) |
| vapor pressure | 1Pa at 280℃ |
| solubility | insoluble in H2O |
| form | powder |
| color | Silver to black |
| Specific Gravity | 5.727 |
| Odor | Odourless |
| Resistivity | 33.3 μΩ-cm |
| Water Solubility | insoluble |
| Sensitive | Air Sensitive |
| Merck | 13,802 |
| Exposure limits | TLV-TWA 0.2 mg(As)/m3 (ACGIH), 0.5 mg (As)/m3 (MSHA), 0.01 mg(As)m3 (OSHA); ceiling 0.002 mg(As)/m3/15 min (NIOSH); carcinogenicity: Human Sufficient Evidence (IARC). |
| Stability | Stable. Incompatible with acids, oxidizing agents, halogens. Heat and air-sensitive. |
| EPA Primary Drinking Water Standard | MCL:0.01,MCLG:0 |
| CAS DataBase Reference | 7440-38-2(CAS DataBase Reference) |
| EWG's Food Scores | 9-10 |
| NCI Dictionary of Cancer Terms | arsenic |
| FDA UNII | N712M78A8G |
| Proposition 65 List | Arsenic (inorganic arsenic compounds) |
| Proposition 65 List | Arsenic (inorganic oxides) |
| IARC | 1 (Vol. 23, Sup 7, 100C) 2012 |
| NIST Chemistry Reference | Arsenic(7440-38-2) |
| EPA Substance Registry System | Arsenic (7440-38-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H315-H318-H350-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P310-P302+P352-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 23/25-50/53-36/38-22-45-52/53-51/53 | |||||||||
| Safety Statements | 20/21-28-45-60-61-26-53 | |||||||||
| RIDADR | UN 1558 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CG0525000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28048000 | |||||||||
| Toxicity | Human exposure occurs occupationally and via food, tobacco smoke, ambient air, and water. Three major groups of arsenic compounds have been defined on the basis of biological considerations: inorganic arsenicals; organic arsenicals; and arsine (gas). The comparative toxicity of these groups is dependent upon the route of exposure and their solubilities; the more quickly absorbed compounds have lower LD50. Arsenic is readily absorbed by the respiratory and gastrointestinal systems and is concentrated in the skin, hair, and nails (Aldrich-Mees’ lines). The cellular toxicity of arsenic is related to reactions with SH-containing mitochondrial enzymes that result in impaired respiration. Arsenic may also compete with phosphate during oxidative phosphorylation. | |||||||||
| IDLA | 5 mg As/m3 | |||||||||
| NFPA 704 |
|
Arsenic price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 267961 | Arsenic powder, ≥99.997% trace metals basis | 7440-38-2 | 10g | $261 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.19773 | Arsenic standard solution traceable to SRM from NIST H?AsO? in HNO? 0.5 mol/l 1000 mg/l As Certipur? | 7440-38-2 | 100ML | $39.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202657 | Arsenic 99.999% trace metals basis | 7440-38-2 | 5g | $97.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.19773 | Arsenic standard solution traceable to SRM from NIST H?AsO? in HNO? 0.5 mol/l 1000 mg/l As Certipur? | 7440-38-2 | 500ML | $55.8 | 2024-03-01 | Buy |
| Alfa Aesar | 000590 | Arsenic lump, 99.999% (metals basis) | 7440-38-2 | 25g | $159 | 2023-06-20 | Buy |
Arsenic Chemical Properties,Uses,Production
Description
Arsenic is a metalloid of the nitrogen group. Two allotrope forms of elemental arsenic have been reported: yellow arsenic and grey arsenic, the latter being usually the more stable form. Arsenic readily oxidises in air to arsenic trioxide (As2O3). Arsenic is mostly found either in its native state or as arsenic sulfide in the form of realgar (As4S4) or orpiment (As2S3). Arsenic can exist in three different valence states (zerovalent, trivalent and pentavalent). Arsenic forms covalent bonds with carbon, oxygen and hydrogen. The toxicity varies widely and depends on the physical state of the compound and its absorption/elimination rate. Trivalent arsenics (As(III)) are derivatives of the arsenous acid (H2AsO3-arsenite) and arsenic trioxide (AsO3). Examples of pentavalent arsenic (As(V)) include derivatives of the arsenic acid (H3AsO4 -arsenate). Organic arsenic-based compounds, that is, compounds containing arsenic-carbon bonds, are usually less toxic than their inorganic counterparts. This is mainly due to their quicker excretion from the human body. Arsenic is known to be one of the most toxic heavy metals. Compounds containing arsenic have a long history of use as poisons, but they also have a long historical medicinal use.
Chemical Properties
Arsenic (As) is the third element in Group VA of the periodic table. Elemental arsenic can be found in two solid forms: yellow and gray or metallic, with specific gravities of 1.97 and 5.73, respectively (CRC, 1999). Gray arsenic is the ordinary stable form. Arsenic compounds can be categorized as inorganic and organic. Inorganic compounds do not contain an arsenic–carbon bond while organic compounds do.

Chemical Properties
Elemental arsenic, as, occurs to a limited extent in nature as a steel-gray, amorphous metalloid.
Physical properties
Arsenic is classed as a semimetal, meaning that it is neither a metal like aluminum or lead,nor quite a nonmetal such as oxygen, sulfur, or chlorine. Arsenic’s main allotrope is a silverygray,brittle, metal-like substance. Its other two isotopes are unstable crystalline substances.
Gray arsenic exhibits an unusual property in that its boiling point (614°C) is lower than itsmelting point (817°C). As its temperature changes, it sublimates, which means it goes fromthe solid state, skipping the liquid state, into a vapor state. Cooling the vapor of sublimation,the black allotrope condenses out and in turn changes from the black to the gray allotrope. Ifyellow arsenic is rapidly cooled from its sublimation point, yellow arsenic will condense outand will not revert back to gray arsenic upon cooling.
The following information is for the gray semimetal form of arsenic only. Its melting pointis 817°C, its sublimation point varies between 613°C and 814°C depending on the atmosphericpressure, and its density is 5.776 g/cm3.
Isotopes
There are a total of 35 isotopes of arsenic, ranging from As-60 to As-92, withhalf-lives spanning from a few nanoseconds to 80 days. Although some references claimthere are no stable isotopes of arsenic, arsenic-75 is classed as a stable isotope thatmakes up 100% of arsenic found in the Earth’s crust.
Origin of Name
Derived either from the Latin word arsenicum or the Greek word arsenikon, both meaning a yellow pigment. It is possible that the Arabic word azzernikh was also an ancient name for arsenic.
Occurrence
Arsenic is the 53rd most abundant element and is widely distributed in the Earth’s crust.It occurs naturally in several minerals, but high-grade deposits are rare. Most of the mineralsand ores that contain arsenic also contain other metals. Some major sources of arsenic are theminerals orpiment, scherbenkobalt, arsenopyrite, niccolite, realgar, gersdorffite, and smaltite.In addition, most sulfide ores of other metals also contain some arsenic. The three major mineralsthat produce arsenic are: realgar (arsenic monosulfide, AsS), orpiment (arsenic trisulfide,As2S2), and arsenopyrite (iron arsenosulfide, FeAsS).
Today, most arsenic is recovered as a by-product from the smelting of nickel, copper, iron,and tin. It is also recovered from the flue dust of copper- and lead-smelting furnaces.
Characteristics
Arsenic in the elemental form is a brittle, grayish crystal that becomes darker when exposedto air. It is seldom found in the pure elemental form but rather in minerals (compounds). Ithas a long history of use as a poison, and many alchemists were poisoned when using it intheir attempts to produce gold from base metals.
Arsenic has limited commercial use.
History
Elemental arsenic occurs in two solid modifications: yellow, and gray or metallic, with specific gravities of 1.97, and 5.75, respectively. Gray arsenic, the ordinary stable form, has a triple point of 817°C and sublimes at 616°C and has a critical temperature of 1400°C. Several other allotropic forms of arsenic are reported in the literature. It is believed that Albertus Magnus obtained the element in 1250 A.D. In 1649 Schroeder published two methods of preparing the element. It is found native, in the sulfides realgar and orpiment, as arsenides and sulfarsenides of heavy metals, as the oxide, and as arsenates. Mispickel, arsenopyrite, (FeSAs) is the most common mineral, from which on heating the arsenic sublimes leaving ferrous sulfide. The element is a steel gray, very brittle, crystalline, semimetallic solid; it tarnishes in air, and when heated is rapidly oxidized to arsenous oxide (As2O3) with the odor of garlic. Arsenic and its compounds are poisonous. Exposure to arsenic and its compounds should not exceed 0.01 mg/m3 as elemental As during an 8-h work day. Arsenic is also used in bronzing, pyrotechny, and for hardening and improving the sphericity of shot. The most important compounds are white arsenic (As2O3), the sulfide, Paris green 3Cu(AsO2)2· Cu(C2H3O2)2, calcium arsenate, and lead arsenate; the last three have been used as agricultural insecticides and poisons. Marsh’s test makes use of the formation and ready decomposition of arsine (AsH3). Arsenic is available in high-purity form. It is finding increasing uses as a doping agent in solid-state devices such as transistors. Gallium arsenide is used as a laser material to convert electricity directly into coherent light. Natural arsenic is made of one isotope 75As. Thirty other radioactive isotopes and isomers are known. Arsenic (99%) costs about $75/50g. Purified arsenic (99.9995%) costs about $50/g.
Uses
Inorganic arsenic compounds were widely used as pesticides from the mid 1800s to the mid 1900s and were used in medicine until the 1970s, primarily for treatment of leukemia, psoriasis, and asthma. The use of arsenic for treatment of acute promyelocytic leukemia resumed in the 1990s. By the mid 1970s, arsenic use was shifting from pesticides to wood preservatives, and by 1980, wood preservatives were the primary use. Total agricultural-chemical use (in pesticides and fertilizers) declined to about 15% to 20% of total arsenic consumption by the early 1990s and has remained at about 4% since 1995 (Edelstein 1994, Reese 1998, ATSDR 2007, Brooks 2009).
Since the mid 1990s, arsenic trioxide used in wood preservation has accounted for 86% to 90% of total U.S. arsenic consumption. Wood treated with chromated copper arsenate (CCA), known as “pressure-treated wood,” has been used widely to protect utility poles, building lumber, and foundations from decay and insect attack. However, a voluntary phase-out of CCA for certain residential uses (e.g., in wood for decks, play structures, fencing, and boardwalks) that went into effect December 31, 2003, has reduced this use of arsenic. CCA continues to be used in wood products for industrial use. Other uses of arsenic in the 1990s included use in glass (3% to 4%) and nonferrous alloys (1% to 4%) (ATSDR 2007, Brooks 2009).
By the 1990s, there was renewed interest in the use of arsenic for treatment of acute promyelocytic leukemia (ATSDR 2007). Arsenic trioxide is approved by the U.S. Food and Drug Administration for treating this type of leukemia when other chemotherapy treatments have failed (MedlinePlus 2009). Arsenic is also used in the production of lead alloys used in lead-acid batteries. It may be added to alloys used for bearings, type metals, lead ammunition, and automotive body solder, and it may be added to brass to improve corrosion resistance. High-purity arsenic is used in a variety of semiconductor applications, including solar cells, light-emitting diodes, lasers, and integrated circuits (ATSDR 2007).
Uses
Over the years a number of practical uses for arsenic developed, particularly related toits poisonous nature. Today, it is not of great commercial value except as an insecticide andherbicide.
It is used in the semiconductor industry to coat solid-state devices. Some compounds areused in paints and fireworks. The major uses are in medicine, where its toxic properties areimportant for the treatment of diseases.
Uses
Arsenic is used for hardening metals suchas copper and lead and as a doping agentin solid-state products of silicon and germanium.Its salts are used in making herbicidesand rodenticides, in semiconductors, and inpyrotechnics. Arsenic trioxide is being usedin experimental research for treating solidtumors such as gastric cancer and head andneck tumors.
Uses
Arsenic is a brittle solid with a metallic coloring that ranges from silver to gray. It is a naturally occurring element found in the earth’s crust, and it cycles rapidly through water, land, air, and living systems. Exposure to it occurs through ingestion, inhalation, and dermal contact.
The arsenic metalloid is used for hardening copper and lead alloys (HSDB, 2005). It is also used in glass manufacturing as a decolorizing and refining agent, as a component of electrical devices in the semiconductor industry, and as a catalyst in the production of ethylene oxide. Arsenic compounds are used as a mordant in the textile industry, for preserving hides, as medicinals, pesticides, pigments, and wood preservatives. The production of chromate copper arsenate (CCA), a wood preservative, accounts for approximately 90% of the domestic arsenic consumption (ATSDR, 2007). However, production of this preservative is being phased out. The uses of inorganic arsenical compounds (e.g., lead arsenate) as pesticides were voluntarily cancelled by the industry during late 1980s and early 1990s. A majority of organoarsenicals are used on cotton and turf as herbicides. disodium methanearsenate (DSMA), monosodium methanearsenate (MSMA), and calcium methanearsenate (CAMA) continue to be used as contact herbicides.
Production Methods
The process most often reported in the litera ture treats white arsenic with hydrochloric acid to form arsenic trichloride: As2O3 +6HCl → 2AsCl3+3H2O
The arsenic trichloride is purified by fractional distillation, possibly in combination with chem ical methods. It subsequently is reduced by re action with hydrogen at 800–850℃, and the metal vapor is condensed in crystalline form at 400–450℃.
4AsCl3 +6H2 → As4+6HCl
After sublimation,the metal is packaged in glass ampoules. An arsenic content of >99.99 % can be obtained by this method.
Definition
arsenic: Symbol As. A metalloid elementof group 15 (formerly VB) ofthe periodic table; a.n. 33; r.a.m.74.92; r.d. 5.7; sublimes at 613°C. Ithas three allotropes-yellow, black,and grey. The grey metallic form isthe stable and most common one.Over 150 minerals contain arsenicbut the main sources are as impuritiesin sulphide ores and in the mineralsorpiment (As2S3) and realgar(As4S4). Ores are roasted in air toform arsenic oxide and then reducedby hydrogen or carbon to metallic arsenic.Arsenic compounds are used ininsecticides and as doping agents insemiconductors. The element is includedin some lead-based alloys topromote hardening. Confusion canarise because As4O6 is often sold aswhite arsenic. Arsenic compoundsare accumulative poisons. The elementwill react with halogens, concentratedoxidizing acids, and hot alkalis.Albertus Magnus is believed tohave been the first to isolate the elementin 1250.
Definition
A toxic metalloid element existing in
several allotropic forms; the most stable is
a brittle gray metal. It belongs to group 15
(formerly VA) of the periodic table. Arsenic
is found native and in several ores including
mispickel (FeSAs), realgar (As4S3),
and orpiment (As2S3). The element reacts
with hot acids and molten sodium hydroxide
but is unaffected by water and acids
and alkalis at normal temperatures. It is
used in semiconductor devices, alloys, and
gun shot. Various compounds are used in
medicines and agricultural insecticides and
poisons.
Symbol: As; m.p. 817°C (gray) at 3
MPa pressure; sublimes at 616°C (gray);
r.d. 5.78 (gray at 20°C); p.n. 33; r.a.m.
74.92159.
General Description
A grayish metallic solid that turns black upon exposure to air. Insoluble in water. Toxic by ingestion.
Air & Water Reactions
Turns black on exposure to air. Insoluble in water.
Reactivity Profile
Arsenic reacts incandescently with bromine trifluoride, even at 10°C [Mellor 2:113 1946-47]. Causes bromoazide to explode upon contact. Ignites if ground up together with solid potassium permanganate [Mellor 12:322 1946-47]. Is oxidized by sodium peroxide with incandescence [Mellor 2:490-93 1946-47]. A combination of finely divided Arsenic with finely divided bromates (also chlorates and iodates) of barium, calcium, magnesium, potassium, sodium, or zinc can explode by heat, percussion, and friction [Mellor 2:310 1946-47]. Bromine pentafluoride reacts readily in the cold with Arsenic. Ignition usually occurs. Reacts vigorously with fluorine at ordinary temperatures [Mellor 9:34 1946-47].
Hazard
Most of the compounds of arsenic are toxic when in contact with the skin, when inhaled,or when ingested. As with arsenic’s cousin phosphorus above it in group 15 of the periodictable, care must be taken when using arsenic. The compound arsenic trioxide (As2O3), anexcellent weed-killer, is also carcinogenic. Copper acetoarsenite, known as Paris green, is usedto spray cotton for boll weevils. A poisonous dose of arsenic as small as 60 milligrams can bedetected within the body by using the Marsh test.
Health Hazard
One of the allotropic forms, yellow arsenic,is a severe human poison. The fatal dosein humans is 1–2 mg/kg body weight. Allarsenic compounds are toxic, the toxicityvarying with the oxidation state of themetal and the solubility. Thus, the trivalentinorganic compounds of arsenic, suchas arsenic trichloride, arsenic trioxide, andarsine, are highly toxic—more poisonousthan the metal and its pentavalent salts.The organic arsenic compound Lewisite isa severe blistering agent that can penetratethe skin and cause damage at the point ofexposure. Lewisite was used as a poison gasin World War I. Less soluble arsenic sulfideexhibits a lower acute toxicity.
Arsenic is absorbed into the body througha GI route and inhalation. The acute symptomsinclude fever, GI disturbances, irritationof the respiratory tract, ulceration of thenasal septum, and dermatitis. Chronic exposurecan produce pigmentation of the skin,peripheral neuropathy, and degeneration ofliver and kidneys.
The toxic effects of arsenic are attributedto its binding properties with sulfur. It formscomplexes with coenzymes. This inhibitsthe production of adenosine triphosphate (ATP), which is essential for energy in bodymetabolism. 2,3-Dimercapto-1-propanol isan antidote against acute intoxication. 2,3-Dithioerythritol is reported to be a moreeffective and less toxic antidote (Boyd et al.1989). Arsenic is carcinogenic to humans.Ingestion by an oral route has caused anincreased incidence of tumors in the liver,blood, and lungs. The mutagenic and genotoxiceffects of arsenic have been reviewed(Basu et al. 2001).
Peraza et al. (2003) studied toxicity andmetabolism of inorganic arsenic in kidneyat low level subcytotoxic concentrations.Human renal proximal tubule epithelial cells(HK-2) were used as model in their study.The authors found that HK-2 cells werecapable of biotransforming inorganic arseniccompounds in a pathway involving reductionof arsenate to arsenite.
Bernstam et al. (2002) measured percutaneousabsorption of As(III) and As(V) invitro using artificial human skin. The permeabilityconstant (Kp) for As(V) and As(III)were determined from this study as 4.3× 10-5 cm/h and 10.1× 10-5 cm/h respectively.As(III) at exposure doses as low as10 μg/L could cause significant morphologicalchanges, disruption of cell membrane andinhibition of deoxyribonucleic acid (DNA)and protein syntheses. The authors noted thatconcentrations of tri- or pentavalent arsenicat levels above 100 μg/L in showering- orhand-washing waters could manifest harmfuleffects.
Flammability and Explosibility
Not classified
Industrial uses
Arsenic (symbol As) is a soft, brittle, poisonouselement of steel-gray color and metallic luster.In atomic structure it is a semimetal, lackingplasticity, and is used only in alloys and incompounds. The bulk of the arsenic used isemployed in insecticides, rat poisons, and weedkillers, but it has many industrial uses, especiallyin pigments. It is also used in poisongases for chemical warfare.Metallic arsenic is stable in dry air. Whenexposed to humid or moistened air, the surfacefirst becomes coated with a superficial goldenbronze tarnish, which on further exposure turnsblack. On heating in air arsenic will vaporizeand burn to As2O3.
Safety Profile
Confirmed human carcinogen producing liver tumors. Poison by subcutaneous, intramuscular, and intraperitoneal routes. Human systemic skin and gastrointestinal effects by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. Flammable in the form of dust when exposed to heat or flame or by chemical reaction with powerful oxidizers such as bromates, chlorates, iodates, peroxides, lithium, NC4, m 0 3 , Khfn04, Rb2C2, AgN04, NOCl, IF5, CrO3, CIF3, Cl0, BrF3, BrFj, BrN3, RbGBCH, CsC3BCH. Slightly explosive in the form of dust when exposed to flame. When heated or on contact with acid or acid fumes, it emits highly toxic fumes; can react vigorously on contact with oxidizing materials. Incompatible with bromine azide, dirubidium acetylide, halogens, palladium, zinc, platinum, NCh, AgNO3, CrO3, Na2O2, hexafluoroisopropylideneamino lithum.
Potential Exposure
Arsenic compounds have a variety of uses. Arsenic and its compounds are used as an alloy additive, in electronic devices; in veterinary medicines; in agriculture as insecticides, herbicides, larvicides, and pesticides. Some arsenic compounds are used in pigment production; the manufacture of glass as a bronzing or decolorizing agent; the manufacture of opal glass and enamels, textile printing; tanning, taxidermy, antifouling paints; to control sludge formation in lubricating oils. Metallic arsenic is used as an alloying agent for heavy metals; and in solders, medicines, herbicides. EPA has estimated that more than 6 million people living within 12 mi of major sources of copper, zinc, and lead smelters-may be exposed to 10 times the average United States atmospheric levels of arsenic. The agency says that 40,000 people living near some copper smelters may be exposed to 100 times the national atmospheric average.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: For severe poisoning BAL [British AntiLewisite, Dimercaprol, dithiopropanol (C3H8OS2)] has beenused to treat toxic symptoms of certain heavy metals poisoning—including arsenic. Although BAL is reported to have alarge margin of safety, caution must be exercised, becausetoxic effects may be caused by excessive dosage. Most canbe prevented by premedication with 1-ephedrine sulfate(CAS: 134-72-5). Oral penicillamine (not penicillin) hasbeen used as a follow-up treatment or used instead of BALfor milder poisoning, with mixed success. Side effects mayoccur with such treatment and it is never a substitute forcontrolling exposure. Treatment can only be done understrict medical care.
Carcinogenicity
Arsenic and inorganic arsenic compounds are known to be human carcinogens based on sufficient evidence of carcinogenicity in humans.
Environmental Fate
Trivalent arsenic exerts its toxic effects mainly by disrupting ATP production by inhibiting lipoic acid, which is a cofactor for pyruvate as well by replacing phosphate which uncouples oxidation phosphorylation. This inhibits the electron transport chain in the mitochondria and the ultimate synthesis of ATP. Hydrogen peroxide production is also increased, which, it is speculated, has potential to form reactive oxygen species and oxidative stress. These metabolic interferences lead to death from multisystem cell death and organ failure. The activity of enzymes is due to the functional groups on amino acids such as the sulfhydryl group on cysteine or coenzymes such as lipoic acid, which has vicinal thiol groups. Trivalent inorganic arsenicals readily react with sulfhydryl groups such as cysteine creating a strong complex between arsenic and vicinal sulfhydryl reagents. These actions inhibit not only the formation of Acetyl-CoA but also the enzymes succinic dehydrogenase and pyruvate. Arsenite inhibits the binding of steroids to the glucocorticoid receptor, but not other steroid receptors. The probable mechanism of toxicity of pentavalent inorganic arsenate is its reduction to a trivalent form, arsenite, which is more toxic than the arsenate. Thus, a variety of mechanisms lead arsenic to impair cell respiration and subsequently diminish ATP formation.
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Arsenic must be stored in a cool, dry place away from oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates) and strong acids (such as hydrochloric,sulfuric, and nitric) since violent reactions occur. A regulated, marked area should be established where this chemicalis handled, used, or stored in compliance with OSHAStandard 1910.1045.
Shipping
UN1558 Arsenic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Toxicity evaluation
Bioconcentration of arsenic occurs in algae and lower invertebrates. Both bottom-feeding and predatory fish can accumulate contaminants found in water. The major bioaccumulation transfer is between water and algae, at the base of the food chain that has a strong impact on the concentration in fish. Bottom-feeders are readily exposed to the greater quantities of arsenic, which accumulate in sediments. No differences were found for arsenic existing between bottom-feeders and predators in tissue levels of metals and other contaminants. Therefore, biomagnification in aquatic food chains does not appear to be significant.
Incompatibilities
Incompatible with strong acids; strong oxidizers; peroxides, bromine azide, bromine pentafluoride, bromine trifluoride; cesium acetylene carbide, chromium trioxide; nitrogen trichloride, silver nitrate. Can react vigorously with strong oxidizers (chlorine, dichromate, permanganate). Forms highly toxic fumes on contact with acids or active metals (iron, aluminum, zinc). Hydrogen gas can react with inorganic arsenic to form highly toxic arsine gas.
Waste Disposal
Elemental arsenic wastes should be placed in long-term storage or returned to suppliers or manufacturers for reprocessing. Arsenic pentaselenide-wastes should be placed in long-term storage or returned to suppliers or manufacturers for reprocessing. Arsenic trichloride: hydrolyze to arsenic trioxide utilizing scrubbers for hydrogen chloride abatement. The trioxide may then be placed in long-term storage. Arsenic trioxide: long-term storage in large shiftproof and weatherproof silos. This compound may also be dissolved, precipitated as the sulfide and returned to the suppliers. Arsenic-containing sewage may be decontaminated by pyrolusite treatment. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Arsenic Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| changzhou huayang technology co., ltd | +8615250961469 | 2571773637@qq.com | China | 9821 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15745 | 58 |
Related articles
- Uses and safety of high-purity arsenic
- The demand for metallic arsenic is limited. It is used in nonferrous alloys, and high-purity arsenic is used in electronic and....
- Mar 16,2024
- Toxicity of Arsenic
- The word arsenic has several derivations including from the Syriac word (al) zarniqa, the Persian word zarnikh,and the Greek w....
- Nov 16,2021
- Arsenic——Exposure Sources & Medical Management
- Arsenic (As; CAS #7440-38-2) is a brittle solid with a metallic coloring that ranges from silver to gray. It is a naturally oc....
- Mar 5,2020
View Lastest Price from Arsenic manufacturers
7440-38-2(Arsenic)Related Search:
1of4