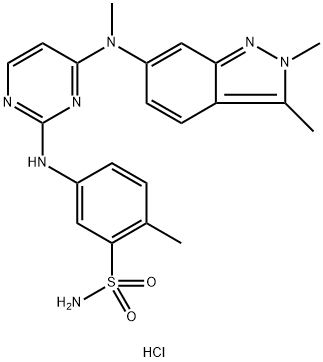
What is the solubility of Pazopanib hydrochloride?
Mar 19,2024
Description
Pazopanib hydrochloride (PZH) is chemically designated as 5-[[4-[(2,3-dimethylindazol-6-yl)-methylamino] pyrimidin-2-yl] amino]− 2-methyl benzene sulfonamide hydrochloride, and chemically formulated as C21H24ClN7O2S. PZH is the second generation's multi-targeted tyrosine kinase inhibitor. The current trademark for PZH is Votrient®. It inhibits tumor growth and angiogenesis as a highly efficient and selective multi-targeted receptor tyrosine kinase inhibitor. PZH is employed in treating renal cell carcinoma and soft tissue sarcoma.
Solubility
PZH was found to exhibit a low aqueous solubility. The intestinal permeability of PZH is considered to be high. Therefore, the drug is classified as a class II compound in the biopharmaceutics classification system (BCS). This implies that its absorption and bioavailability is primarily hindered by solubility.
The commercial formulation of PZH consists of a physical mixture of microcrystalline cellulose, povidone K30, magnesium stearate, and sodium starch glycolate in the form of an immediate-release tablet. The oral bioavailability of 800 mg of the commercial formulation was found to have a median of 21%. Pazopanib exhibits a relatively large inter-patient variability in both exposure and plasma concentrations. The intra-patient variability in these pharmacokinetic parameters was found to be similarly large. This large variability may be related to the variable absorption process of pazopanib.
Accordingly, at temperatures (308–338 K) and pressures (120–270 bar), the solubility of the anticancer drug pazopanib hydrochloride (PZH) in supercritical carbon dioxide (Sc-CO2) was experimentally investigated. PZH dissolved (in the Sc-CO2 mole fractions) ranged from 1.87 × 10−6 to 14.25 × 10−6, resulting in solubilities between 0.015 and 0.120 g/L.
According to Nuijen et al., the one containing the co-block polymer Soluplus® in an 8:1 ratio with PZH performed best regarding in vitro dissolution properties. The in vivo results indicated that 300 mg of the developed formulation yields similar exposure and a lower variability (379 μg/mL*h (36.7% CV)) than previously reported values for the standard PZH formulation (Votrient®) at the approved dose of 800 mg. Furthermore, the expected plasma-Cthrough levels (27.2 μg/mL) exceed the defined therapeutic efficacy threshold of 20 μg/mL.
References
[1] Sodeifian, G. et al. “Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling.” The Journal of Supercritical Fluids 55 1 (2022): 0.
[2] Maikel Herbrink . “Solubility and bioavailability improvement of pazopanib hydrochloride.” International Journal of Pharmaceutics 544 1 (2018): Pages 181-190.
- Related articles
- Related Qustion
- What are the uses of Pazopanib Hydrochloride? Jul 29, 2025
Pazopanib Hydrochloride is a novel oral, potent and highly selective multi-target multi-protein tyrosine kinase small molecule inhibitor with potential anti-tumor activity.
- Synthesis, Detection and Bioactivity of Pazopanib Hydrochloride Sep 13, 2022
Pazopanib Hydrochloride is an oral angiogenesis inhibitor targeting VEGFR and PDGFR.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringSilicon dioxide is a naturally occurring compound. It consists of silicon and oxygen. The most common form is quartz, which occurs naturally in water, plants, animals and the Earth's crust.....
Mar 19,2024APIPazopanib Hydrochloride
635702-64-6You may like
Pazopanib Hydrochloride manufacturers
- GW786034B
-
- $48.00 / 10mg
- 2025-12-10
- CAS:635702-64-6
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g
- Pazopanib Hydrochlorid
-

- $0.00 / 1g
- 2025-09-11
- CAS:635702-64-6
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
- Pazopanib Hydrochloride
-

- $0.00 / 1g
- 2025-09-11
- CAS:635702-64-6
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 50kg/Month