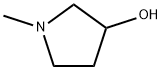
1-Methyl-3-pyrrolidinol synthesis
- Product Name:1-Methyl-3-pyrrolidinol
- CAS Number:13220-33-2
- Molecular formula:C5H11NO
- Molecular Weight:101.15
2419-74-1
56 suppliers
$45.00/100mg
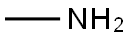
74-89-5
0 suppliers
$13.44/25ML

13220-33-2
319 suppliers
$11.00/1g
Yield:13220-33-2 64.8%
Reaction Conditions:
in water at 10 - 120; under 7500.75 Torr; for 10 h;Autoclave;Sealed tube;Temperature;Pressure;
Steps:
5 (Example 5)
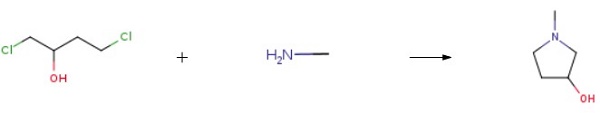
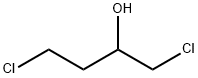
This embodiment is 3-hydroxy-1-methyl tetrahydropyrrole preparation method, the specific steps are as follows: A 500 mL four-necked flask was charged with 250 g of a 40 wt% aqueous solution of monomethylamine and cooled to 10 ° C in an ice-water bath. Then, while stirring, 102 g of 1,4-dichloro-2-butanol was added dropwise and the temperature was controlled at 15 ° C The following, about 15min drip finished; Then pour the system into 500mLAutoclave, sealed, evacuated to a pressure of 1.0 ± 0.1MPa, while heated to 120 ± 2 , the reaction was stirred for about 10h, GC control of raw materials disappear. After the reaction was over, cooled to room temperature, the material was discharged, batchwise add 110g of sodium hydroxide, release a large amount of methylamine gas, control the temperature below 50 , precipitated a lot of white solid, stirred for 1h; filtration, the filtrate layered, the upper organic phase Ethanol 100mLAnd anhydrous magnesium sulfate 18g, stirring 2 ~ 3h; and then filtered, the filtrate was concentrated in vacuo to give a yellow transparent oily liquid, and then vacuum distillation to give 46.7g of colorless and transparent 3-hydroxy-1-methyl tetrahydropyrrole , Yield 64.8%, purity 99.3% (HPLC).
References:
Changzhou Sunshine Pharmaceutical Co., Ltd.;Hu Jinping;Hu Guoyi;Zheng Jianlong;Xi Xiaojin CN106631956, 2017, A Location in patent:Paragraph 0022; 0023; 0024; 0025; 0026; 0027
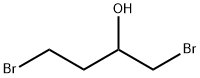
19398-47-1
95 suppliers
$27.00/5g
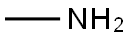
74-89-5
0 suppliers
$13.44/25ML

13220-33-2
319 suppliers
$11.00/1g
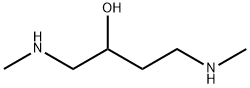
1404453-61-7
0 suppliers
inquiry
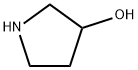
40499-83-0
211 suppliers
$14.00/1g
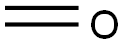
50-00-0
896 suppliers
$10.00/25g

13220-33-2
319 suppliers
$11.00/1g

19398-47-1
95 suppliers
$27.00/5g
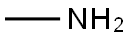
74-89-5
0 suppliers
$13.44/25ML

13220-33-2
319 suppliers
$11.00/1g
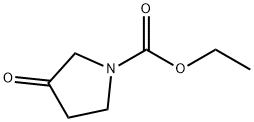
14891-10-2
114 suppliers
$15.00/250mg

13220-33-2
319 suppliers
$11.00/1g