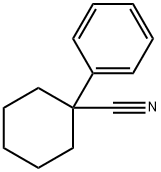
1-Phenyl-1-cyclohexanecarbonitrile synthesis
- Product Name:1-Phenyl-1-cyclohexanecarbonitrile
- CAS Number:2201-23-2
- Molecular formula:C13H15N
- Molecular Weight:185.26

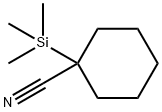
111-24-0
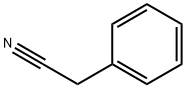
140-29-4

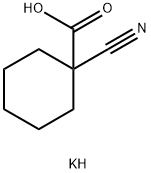
2201-23-2
GENERAL STEPS: A mixture of benzeneacetonitrile (50.0 g, 426.8 mmol) and 1,5-dibromopentane (58.1 mL, 426.8 mmol) was added slowly and dropwise to a dimethylsulfoxide (600.0 mL) suspension of NaH (42.7 g, 1067.0 mmol, 60%) at 0 °C. The mixture was pre-dissolved in a solvent mixture of dimethyl sulfoxide and ether (1:1, 200.0 mL). After dropwise addition, the reaction mixture was continued to be stirred at 0°C for 2 hours. Upon completion of the reaction, water and 10% HCl solution were added to the mixture, followed by extraction with ethyl acetate. The organic layers were combined, washed sequentially with water and brine and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product. The crude product was purified by conventional silica gel column chromatography (eluent: hexane) to afford 1-phenyl-1-cyclohexanecarbonitrile (52.0 g, yield 65.76%) as a colorless oil.GC-MS analysis showed that the molecular ion peak m/z was 185.0.
Yield: 89.5%
Reaction Conditions:
Stage #1:phenylacetonitrile with sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2:1,5-dibromo-pentane in N,N-dimethyl-formamide at 20; for 4 h;
Steps:
49 Synthesis of 1-phenylcyclohexane-1-nitrile (49A)
Dissolve NaH (2.05g, 85.36mmol) in DMF (10mL), slowly add benzylacetonitrile (2.00g, 17.07mmol) dropwise at 0, stir for 0.5h, add 1,5-dibromopentane (3.93g , 17.07mmol), react at room temperature for 4h, add 80mL water, extract with ethyl acetate (25mL×4), combine the organic layers, wash with saturated brine, dry with anhydrous magnesium sulfate, filter with suction, concentrate under reduced pressure, and separate and purify by column chromatography , To obtain 2.83 g of colorless oil, yield 89.5%
References:
CN113061098, 2021, A Location in patent:Paragraph 0399-0402
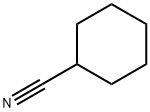
766-05-2
146 suppliers
$13.43/1gm:
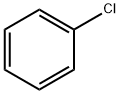
108-90-7
685 suppliers
$10.00/25G

2201-23-2
92 suppliers
$39.00/1 g
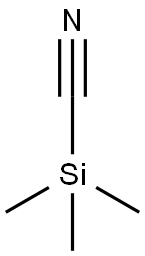
7677-24-9
394 suppliers
$19.00/5g
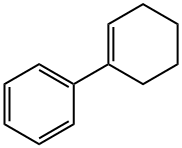
771-98-2
151 suppliers
$10.00/1g

2201-23-2
92 suppliers
$39.00/1 g