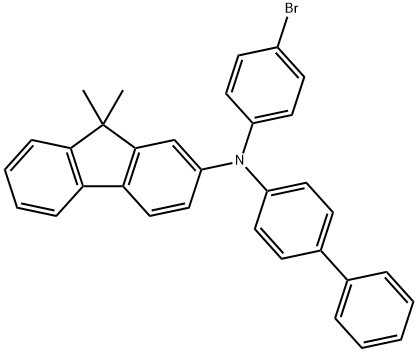
N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine synthesis
- Product Name:N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine
- CAS Number:1246562-40-2
- Molecular formula:C33H26BrN
- Molecular Weight:516.47
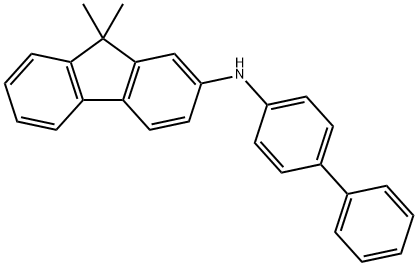
897671-69-1
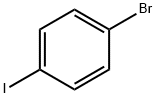
589-87-7
![N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine](/CAS/GIF/1246562-40-2.gif)
1246562-40-2
GENERAL STEPS: A mixture of N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-2-amine (1.00 g, 2.77 mmol), 1-bromo-4-iodobenzene (1.00 g, 3.53 mmol), potassium hydroxide (0.30 g, 5.38 mmol), cuprous iodide (5.70 mg, 0.03 mmol) and 1,10- Phenanthroline monohydrate (5.40 mg, 0.03 mmol) in o-xylene (30 mL) was reacted with stirring under nitrogen protection at 150 °C for 8 hours. After completion of the reaction, it was cooled to room temperature and the reaction solution was concentrated under reduced pressure. Cold water was added to the residue, which was then extracted with dichloromethane (DCM). The organic phases were combined, filtered and dried over anhydrous magnesium sulfate (MgSO4). The crude product was purified by silica gel (SiO2) column chromatography with the eluent of petroleum ether/dichloromethane (4:1, v/v) to afford the target product N-[1,1'-biphenyl]-4-yl-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine (1.31 g, 91.8% yield).1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 7.5 Hz, 1H), 7.52 (t, J = 7.5 Hz, 3H), 7.43 (d, J = 8.7 Hz, 2H), 7.35 (dd, J = 15.4, 7.4 Hz, 3H), 7.29 (d, J = 8.9 Hz, 2H), 7.23 (m, 3H), 7.14 (d, J = 2.0 Hz, 1H). 7.10 (d, J = 8.6 Hz, 2H), 6.98 (m, 3H), 1.36 (s, 6H).

897671-69-1
249 suppliers
$6.00/250mg

589-87-7
540 suppliers
$10.00/1g
![N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine](/CAS/GIF/1246562-40-2.gif)
1246562-40-2
140 suppliers
$15.00/1g
Yield:1246562-40-2 91.8%
Reaction Conditions:
with copper(l) iodide;1,10-Phenanthroline;potassium hydroxide in o-xylene;water at 150; for 8 h;Inert atmosphere;Ullmann Condensation;
Steps:
4.2.1 Synthesis of N-([1,1'-biphenyl]-4-yl)-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine (1)
A mixture of N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-2-mine (1.00 g, 2.77 mmol), 1-bromo-4-iodobenzene (1.00 g, 3,53 mmol), potassium hydroxide (0.30 g, 5.38 mmol), coprous iodide (5.70mg, 0.03 mmol) and 1,10-Phenanthroline monohydrate (5.40 mg, 0.03 mmol) in ortho-xylene (30 mL) was stirred at 150 °C for 8 h under nitrogen atmosphere. After cooling to room temperature, the solution was evaporated in vacuum. The cold water was added to the mixture and finally extracted with DCM. The combined organic phase was collected, filtered and dried over MgSO4. The crude product was purified by SiO2 column chromatography using petroleum ether/dichloromethane (4:1, v/v) afforded 1 (1.31 g, 91.8 %). 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J=7.5 Hz, 1H), 7.52 (t, J=7.5 Hz, 3H), 7.43 (d, J=8.7 Hz, 2H), 7.35 (dd, J=15.4, 7.4 Hz, 3H), 7.29 (d, J=8.9 Hz, 2H), 7.23 (m, 3H), 7.14 (d, J=2.0 Hz, 1H), 7.10 (d, J=8.6 Hz, 2H), 6.98 (m, 3H), 1.36 (s, 6H).
References:
Wu, Panpan;Tian, Guojian;Hu, Mingming;Lian, Hong;Dong, Qingchen;Liang, Wenting;Huang, Jinhai;Su, Jianhua [Tetrahedron,2017,vol. 73,# 31,p. 4610 - 4615]

897671-69-1
249 suppliers
$6.00/250mg
106-37-6
478 suppliers
$9.00/5g
![N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine](/CAS/GIF/1246562-40-2.gif)
1246562-40-2
140 suppliers
$15.00/1g

897671-69-1
249 suppliers
$6.00/250mg
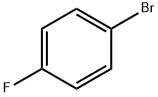
460-00-4
636 suppliers
$6.00/10g
![N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine](/CAS/GIF/1246562-40-2.gif)
1246562-40-2
140 suppliers
$15.00/1g

897671-69-1
249 suppliers
$6.00/250mg
![N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine](/CAS/GIF/1246562-40-2.gif)
1246562-40-2
140 suppliers
$15.00/1g
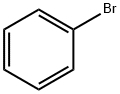
108-86-1
523 suppliers
$10.00/5g
![N-[1,1'-biphenyl]-4-yl-N-(4-broMophenyl)-9,9-diMethyl-9H-Fluoren-2-aMine](/CAS/GIF/1246562-40-2.gif)
1246562-40-2
140 suppliers
$15.00/1g