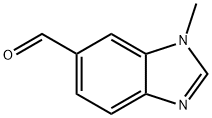
1H-Benzimidazole-6-carboxaldehyde, 1-methyl- (9CI) synthesis
- Product Name:1H-Benzimidazole-6-carboxaldehyde, 1-methyl- (9CI)
- CAS Number:181867-19-6
- Molecular formula:C9H8N2O
- Molecular Weight:160.17

64-18-6
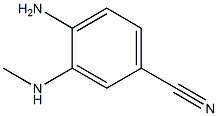
64910-49-2

181867-19-6
(d) Synthesis of 1-methyl-1H-benzimidazole-6-carbaldehyde: 4-amino-3-(methylamino)benzonitrile (0.40 g, 2.72 mmol) was dissolved in formic acid (9 mL) and the mixture was heated to 100 °C and maintained for 2 hours. After the reaction was completed, the mixture was cooled to room temperature, nickel ruanne (0.4 g) and water (2 mL) were added, and the mixture was again heated to 100 °C and maintained for 1 hour. Subsequently, the reaction mixture was filtered through diatomaceous earth while hot, the filter cake was washed with methanol, the filtrates were combined and concentrated under reduced pressure. Water (1 mL) was added to the concentrated residue and saturated sodium bicarbonate solution was added slowly and dropwise to neutral. The precipitated solid was collected by filtration, washed with water and dried to afford 1-methyl-1H-benzimidazole-6-carbaldehyde (0.412 g, 95% yield) as a tan solid, which was used directly in the subsequent reaction.1H NMR (CDCl3) data: δ 10.12 (s, 1H), 8.05 (s, 1H), 8.00 (d, J = 0.8 Hz, 1H), and 7.92 (d, J = 8.0 Hz, 1H), 7.84 (dd, J = 1.2, 8.0 Hz, 1H), 3.94 (s, 3H).

64-18-6
1132 suppliers
$22.12/250ML

64910-49-2
6 suppliers
inquiry

181867-19-6
34 suppliers
$175.00/50mg
Yield:181867-19-6 95%
Reaction Conditions:
Stage #1: formic acid;4-amino-3-(methylamino)benzonitrile at 100; for 2 h;
Stage #2: with water;Raney nikel at 100; for 1 h;
Steps:
1.d
(d) 1-Methyl-lH-benzimidazole-6-carbaldehyde; A mixture of 4-amino-3- (methylamino) benzonitrile (0.40 g, 2.72 mmol) in HCO2H (9 mL) was heated to 100 °C for 2 h. The mixture of crude benzimidazole was then cooled, Raney nickel (0.4 g) and H2O (2 mL) were added, and the mixture was heated again to 100°C for 1 h. The hot mixture was then filtered through Celite, rinsed with MeOH and concentrated under reduced pressure. Water (1 mL) was added to the residue, which was then treated carefully with sat. aq. NaHCO3. The solid which precipitated was filtered, rinsed with H20 and dried to afford 1-methyl-lH-benzimidazole-6-carbaldehyde (0.412 g, 95%) as a tan solid, which was used directly in the next reaction. lH NMR (CDCl3) : No. 10.12 (s, 1H), 8.05 (s, 1H), 8.00 (d, J= 0. 8 Hz, 1H), 7.92 (d, J= 8.0 Hz, 1H), 7.84 (dd, J= 1. 2, 8. 0 Hz, 1H), 3.94 (s, 3H).
References:
WO2005/82901,2005,A1 Location in patent:Page/Page column 40
813449-02-4
0 suppliers
inquiry

181867-19-6
34 suppliers
$175.00/50mg
7790-28-5
395 suppliers
$14.56/25G

813449-02-4
0 suppliers
inquiry

181867-19-6
34 suppliers
$175.00/50mg

53484-14-3
3 suppliers
inquiry

181867-19-6
34 suppliers
$175.00/50mg
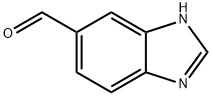
58442-17-4
101 suppliers
$42.00/100mg

74-88-4
357 suppliers
$15.00/10g

181867-19-6
34 suppliers
$175.00/50mg
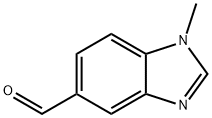
279226-70-9
64 suppliers
$167.00/100mg