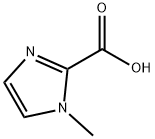
1-Methyl-1H-imidazole-2-carboxylic acid synthesis
- Product Name:1-Methyl-1H-imidazole-2-carboxylic acid
- CAS Number:20485-43-2
- Molecular formula:C5H6N2O2
- Molecular Weight:126.11
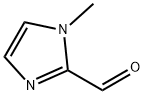
13750-81-7

20485-43-2
The general procedure for the synthesis of 1-methyl-1H-imidazole-2-carboxylic acid from 1-methyl-1H-imidazole-2-carboxaldehyde is as follows: first, 1-methyl-1H-imidazole-2-carboxaldehyde was prepared by referring to the method described in literature [23]. Subsequently, the synthetic procedure for Im2COOH (1b) was followed, and the reaction yield was up to quantitative levels after removal of water under high vacuum (without diethyl ether/water washing). Note that heating may cause decarboxylation to occur. Product yield: 100%. Melting point range is 99-101 °C. NMR hydrogen spectrum (400MHz, D2O): δH 7.42, 7.39 (2H, single peak, imidazolium cyclic-H), 4.08 ppm (3H, single peak, N-CH3); NMR carbon spectrum (400MHz, D2O): δC 158.67, 139.68, 125.83, 118.46, 36.73 ppm. infrared spectrum ( KBr press method): ν 3347 (medium intensity), 3119 (medium intensity), 2663 (weak), 1641 (strong), 1683 (medium intensity), 1507 (strong), 1449 (medium intensity), 1388 (strong), 1338 (strong), 1285 (strong), 1173 (medium intensity), 1123 (strong), 961 (medium intensity), 910 (medium), 776 (strong), 685 (strong) cm-1. Calculated values from elemental analysis (C5H8N2O3, molecular weight 144.12): C, 41.67%; H, 5.59%; N, 19.44%. Measured values: C, 41.28%; H, 5.23%; N, 19.12%.

13750-81-7
244 suppliers
$6.00/250mg

20485-43-2
178 suppliers
$19.00/250mg
Yield:20485-43-2 100%
Reaction Conditions:
with dihydrogen peroxide in water at 20; for 72 h;
Steps:
MeIm2COOH (1c)
First, 1-methylimidazole-2-carboxaldehyde was prepared according to the literature procedure[23]. The synthesis procedure then followed that of Im2COOH (1b) above and the yield was also quantitative after the removal of water under high vacuum (no washing with diethylether/water was necessary). Note: heating causes decarboxylation. Yield: 100%. Mp=99-101°C. δH (400MHz, D2O): 7.42, 7.39 (2H, s, Im-H) and 4.08ppm (3H, s, NCH3); δC (400MHz, D2O): 158.67, 139.68, 125.83, 118.46, 36.73ppm. IR ν (KBr): 3347(m) 3119(m), 2663(w), 1641(s), 1683(m), 1507(s), 1449(m), 1388(s), 1338(s), 1285(s), 1173(m), 1123(s), 961(m) 910(m), 776(s), 685(s) cm-1. Anal. Calc. for C5H8N2O3 (144.12); C, 41.67; H, 5.59; N, 19.44%. Found: C, 41.28; H, 5.23; N, 19.12%.
References:
Gundhla, Isaac Z.;Walmsley, Ryan S.;Ugirinema, Vital;Mnonopi, Nandipha O.;Hosten, Eric;Betz, Richard;Frost, Carminita L.;Tshentu, Zenixole R. [Journal of Inorganic Biochemistry,2015,vol. 145,p. 11 - 18]
30148-21-1
154 suppliers
$27.00/1g

20485-43-2
178 suppliers
$19.00/250mg
541-41-3
0 suppliers
$19.39/5g

20485-43-2
178 suppliers
$19.00/250mg
616-47-7
768 suppliers
$10.00/100g

124-38-9
134 suppliers
$175.00/23402

20485-43-2
178 suppliers
$19.00/250mg