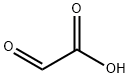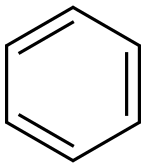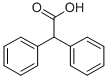
2,2-Diphenylacetic acid synthesis
- Product Name:2,2-Diphenylacetic acid
- CAS Number:117-34-0
- Molecular formula:C14H12O2
- Molecular Weight:212.24
Yield:117-34-0 79%
Reaction Conditions:
with poly(4-vinylpyridine) supported trifluoromethanesulfonic acid at 0 - 20; for 12 h;
Steps:
4.2 General procedure for the condensation reaction of glyoxylic acid with aromatics, using PVP-TfOH(1:10) as the solid acid catalyst
General procedure: The glyoxylic acid, 50 % (0.6 g, 4 mmol), was mixed with arenes (5 mL) in a Nalgene bottle and cooled to 0 °C. Then PVP-TfOH (4.5 g, 28 mmol, 7 equivalents) was added slowly. The reaction mixture was warmed up to room temperature and stirred over specific period of time (Table 1). The reaction progress was monitored by TLC (3:1 hexanes/ethyl acetate). After the reaction was complete, the mixture was poured over ice (25 g), neutralized with sodium bicarbonate, and extracted with diethyl ether (3×15 mL). The organic extracts were combined, washed with water and dried over anhydrous Na2SO4. The solvent was removed by vaccum evaporation and crude products were purified by column chromatography on silica gel (70-230 mesh) using hexane/ethyl acetate (85:15) as eluent. The products were characterized by spectral analysis and by comparing the spectral data with those of the authentic samples (in the case of known compounds). Some of the reactions were also repeated in presence of TfOH (7 equivalents, Table 2). Since dichlorobenzene is a solid aromatic compound, it was dissolved in dichloromethane and the solution was used in the reaction.
References:
Prakash, G.K. Surya;Paknia, Farzaneh;Kulkarni, Aditya;Narayanan, Arjun;Wang, Fang;Rasul, Golam;Mathew, Thomas;Olah, George A. [Journal of Fluorine Chemistry,2015,vol. 171,p. 102 - 112]
947-91-1
124 suppliers
$24.73/1gm:

117-34-0
304 suppliers
$6.00/10g
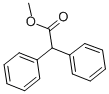
3469-00-9
108 suppliers
$21.00/5g

117-34-0
304 suppliers
$6.00/10g
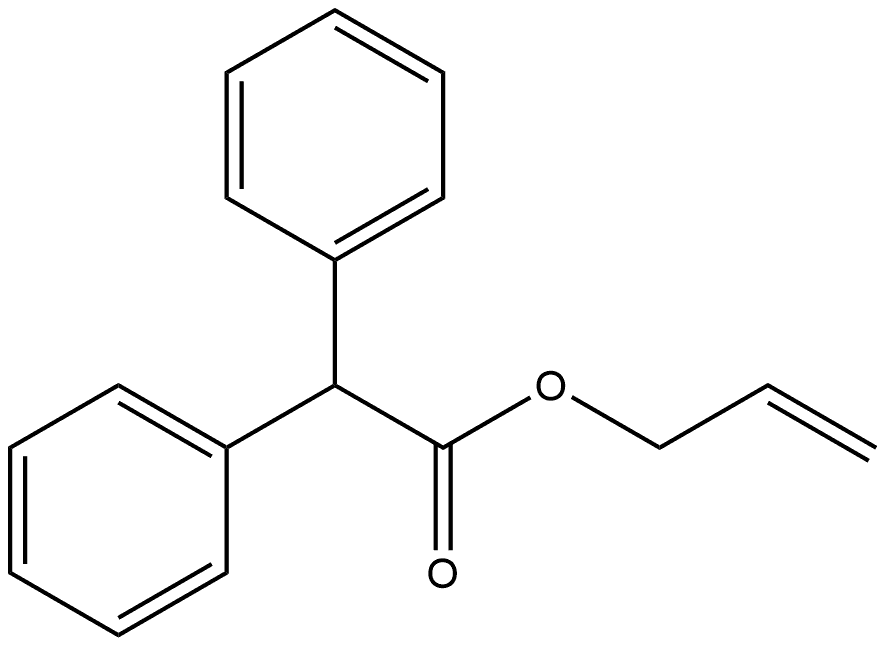
88017-70-3
0 suppliers
inquiry

117-34-0
304 suppliers
$6.00/10g

124-38-9
134 suppliers
$175.00/23402
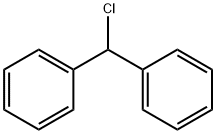
90-99-3
302 suppliers
$9.00/5g

117-34-0
304 suppliers
$6.00/10g