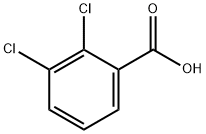
2,3-Dichlorobenzoic acid synthesis
- Product Name:2,3-Dichlorobenzoic acid
- CAS Number:50-45-3
- Molecular formula:C7H4Cl2O2
- Molecular Weight:191.01
Another synthesis of 2,3-dichlorobenzoic acid:
Step (a) A solution of 2,3-dichloroiodobenzene in sodium dried ether was added dropwise to magnesium turnings and a crystal of iodine with warming so as to form a Grignard reagent.
Step (b) The mixture of Step (a) was stirred and refluxed then cooled and transferred dropwise, under nitrogen, into a stirred mixture of sodium dried ether containing solid carbon dioxide.
Step (c) The mixture of Step (b) was stirred for 2 hours, left overnight to warm to room temperature, then treated with ice and aqueous hydrochloric acid, and the product extracted with ether. The combined ether extracts were washed with water then repeatedly extracted with an aqueous sodium hydroxide. These basic solutions were combined, stirred with activated charcoal for 10 minutes, filtered and the cooled filtrate was acidified with concentrated hydrochloric acid at lO.degree. C.
Step (d) The resultant solid was filtered off, washed with water (2.times.20 mis) and dried in vacuo. Yield 77.6%.
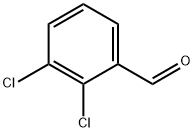
6334-18-5
420 suppliers
$6.00/10g

50-45-3
427 suppliers
$7.00/10g
Yield:-
Reaction Conditions:
Stage #1:2,3-dichlorobenzylaldehyde with ammonia at -33; for 1 h;Inert atmosphere;
Stage #2: with potassium permanganate for 1 h;Inert atmosphere;Reflux;
Steps:
General Procedure for the Oxidation of Aldehydes to Amidesin Liquid Ammonia
General procedure: Under an argon atmosphere, liquid NH3 (25 mL) was condensedin a two-neck round-bottom flask immersed in a dry ice coolingbath and equipped with a dry ice reflux condenser. Aldehyde (7.34 mmol) was added, and the resulting solution (or suspension)was stirred for 1 h. KMnO4 (7.34 mmol, 1.16 g) was added,the cooling bath was removed, and the reaction mixture wasstirred for another hour with gentle reflux of NH3. Na2SO3 (22.0mmol, 2.78 g) was added, the reflux condenser was removed,and the NH3 was allowed to evaporate spontaneously. The darkbrownresidue was treated with 6 M HCl (30 mL), and theresulting precipitate was filtered, washed with H2O (100 mL)and sat. aq NaHCO3 (20 mL). All products were recrystallizedfrom EtOH.
References:
Antoniak, Damian;Sakowicz, Arkadiusz;Loska, Rafał;Makosza, Mieczysław [Synlett,2015,vol. 26,# 1,art. no. ST-2014-D0658-L,p. 84 - 86] Location in patent:supporting information
32768-54-0
267 suppliers
$9.00/25g

50-45-3
427 suppliers
$7.00/10g
6574-97-6
221 suppliers
$6.00/1g

50-45-3
427 suppliers
$7.00/10g
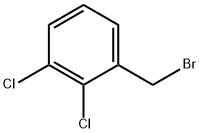
57915-78-3
109 suppliers
$9.00/1g

50-45-3
427 suppliers
$7.00/10g

124-38-9
134 suppliers
$175.00/23402
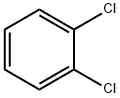
95-50-1
601 suppliers
$10.00/5g

50-45-3
427 suppliers
$7.00/10g