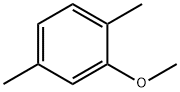
2,5-DIMETHYLANISOLE synthesis
- Product Name:2,5-DIMETHYLANISOLE
- CAS Number:1706-11-2
- Molecular formula:C9H12O
- Molecular Weight:136.19
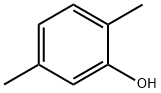
95-87-4

77-78-1

1706-11-2
General procedure for the synthesis of 2,5-dimethylanisole from 2,5-dimethylphenol and dimethyl sulfate: Step 1: 2,5-dimethylphenol (50 g, 410 mmol) and potassium carbonate (68 g, 490 mmol) were dissolved in acetone (600 mL) at room temperature, followed by the addition of dimethyl sulfate (31.02 g, 246 mmol). The reaction mixture was heated and refluxed for 9 h. The progress of the reaction was monitored by TLC and it was found that the starting material was still present. Dimethyl sulfate (31.02 g, 246 mmol) was added additionally and the reaction was continued to reflux for 9 hours. Upon completion of the reaction, the mixture was filtered and acetone was removed using a rotary evaporator. The resulting oil was stirred with 20% sodium hydroxide solution (100 mL) for 10 minutes. The organic layer was washed with water (2 x 500 mL) until the aqueous layer was neutral. The organic layer was dried over sodium sulfate and concentrated in vacuum to give 45.5 g of 2-methoxy-1,4-dimethylbenzene in 81% yield. 1H NMR (CDCl3) δ ppm: 2.2 (3H, s, CH3), 2.34 (3H, s, CH3), 3.82 (3H, s, OCH3), 6.65 (1H, s, Ar-H), 6.7 (1H, d, J = 7.2Hz, Ar-H), 7.03 (1H, d, J = 7.2Hz, Ar-H).
Yield: 81%
Reaction Conditions:
with potassium carbonate in acetone at 20;Reflux;
Steps:
1B.1
Preparation IB) dimethyl 2-amino-5-(methyloxy)-l,4-benzenedicarboxvlate; Step 1:; To a mixture of 2,5-dimethyl phenol (50 g, 410 mmole), and potassium carbonate(68 g; 490 mmole) in acetone (600 ml), dimethyl sulfate (31.02g, 246 mmole) was added at ambient temperature. The mixture was refluxed for 9 h after which TLC revealed presence of the starting material. Additional dimethyl sulfate (31.02 g, 246 mmole) was added and the reaction mixture was refluxed for another 9 h. The reaction mixture was filtered and acetone was removed on a rotavap. The resulting oil was stirred with 20% NaOH (100 ml) for 10 minutes. The organic layer was washed with water (2 X 500 ml) till the aqueous layer was neutral. The organic layer was dried over sodium sulfate and concentrated under vacuum to yield 45.5 g of 2-methoxy- 1 ,4- dimethylbenzene (81%). 1H NMR in CDCl3 δ ppm: 2.2 (3H, s, CH3), 2.34 (3H, s, CH3), 3.82 (3H, s, OCH3), 6.65 (IH, s, Ar-H), 6.7 (IH, d, J = 7.2 Hz, Ar-H), 7.03 (IH, d, J = 7.2 Hz, Ar-H).
References:
GLAXOSMITHKLINE LLC;SCHULZ, Mark, James;WANG, Yonghui WO2010/59549, 2010, A1 Location in patent:Page/Page column 18-19
186583-59-5
33 suppliers
$85.00/250mg

616-38-6
715 suppliers
$5.00/5 g
21573-36-4
24 suppliers
inquiry

1706-11-2
131 suppliers
$11.00/1g

132907-18-7
0 suppliers
inquiry
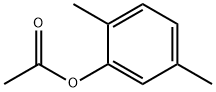
877-48-5
24 suppliers
$181.00/500mg
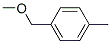
3395-88-8
4 suppliers
inquiry
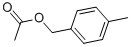
2216-45-7
121 suppliers
$6.00/5g

1706-11-2
131 suppliers
$11.00/1g

