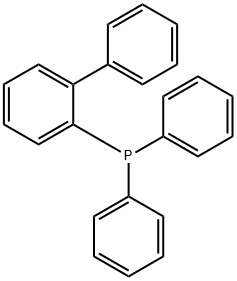
2-(Diphenylphosphino)-biphenyl synthesis
- Product Name:2-(Diphenylphosphino)-biphenyl
- CAS Number:13885-09-1
- Molecular formula:C24H19P
- Molecular Weight:338.38
2113-51-1
829-85-6

13885-09-1
General procedure for the synthesis of 2-(diphenylphosphino)-biphenyl from 2-iodobiphenyl and diphenylphosphine: MCM-41-3N-Pd(0) catalyst (21 mg, 0.01 mmol), KOAc (1.5 mmol), and 2-iodobiphenyl (1.0 mmol if solid) were placed in a 20 mL Schlenk tube that had been pre oven-dried. The reaction vessel was evacuated and displaced three times with argon. Subsequently, 2-iodobiphenyl (1.0 mmol, if liquid), diphenylphosphine (1.2 mmol) and DMAc (1 mL) were added via syringe under argon protection. The reaction mixture was stirred at 130°C for 3 hours. After the reaction was completed, the mixture was cooled to room temperature, diluted with CH2Cl2 (20 mL) and filtered.The MCM-41-3N-Pd(0) catalyst was washed with distilled water (2 × 5 mL) and ethanol (2 × 5 mL) for next use. The filtrate was concentrated under vacuum and the residue was purified by fast column chromatography on silica gel to afford the target product 2-(diphenylphosphino)-biphenyl.
2052-07-5
397 suppliers
$16.30/1g
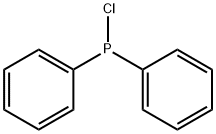
1079-66-9
449 suppliers
$5.00/5g

13885-09-1
186 suppliers
$6.00/250mg
Yield:13885-09-1 79%
Reaction Conditions:
Stage #1:2-Bromobiphenyl with n-butyllithium in diethyl ether;hexane at -15;Inert atmosphere;
Stage #2:chloro-diphenylphosphine in diethyl ether;hexane at 20; for 3 h;Cooling;Inert atmosphere;
Steps:
17 Reference Example 17
Under argon atmosphere, 2-bromobiphenyl (1.40 g, 6.00 mmol) was dissolved into diethyl ether (10 mL), and [the obtainment] was cooled down to -15° C.
In to this solution, n-butyllithium in a 2.67M hexane solution (2.4 mL, 6.41 mmol) was delivered by drops, and after stirring for 2 hours, chlorodiphenylphosphine in a 0.54M diethyl ether solution (10 mL, 5.4 mmol) was added, and [the mixture] was slowly heated to room temperature and stirred for 3 hours.
The reaction liquid was filtrated, and the filtrate was concentrated under reduced pressure, and the obtained crude product was refined by silica gel column chromatography (eluent: hexane/ethyl acetate), and then, a white solid of (2-biphenyl) diphenyl phosphine was obtained (yield amount: 1.6 g, yield: 79%).
1H-NMR (400 MHz, CDCl3), δ (ppm): 7.41˜7.37 (m, 1H), 7.34˜7.16 (m, 17H), 7.07˜7.04 (m, 1H).
31P-NMR (162 MHz, CDCl3), δ (ppm): -13.5 (s).
References:
TOSOH CORPORATION;SAGAMI CHEMICAL RESEARCH INSTITUTE;ARAKI, Keisuke;HONDA, Hiroya;KOISO, Naoyuki;NAKAGAME, Ryo;YOSHITOMI, Fumiaki;IWANAGA, Kohei;FURUKAWA, Taishi US2020/354389, 2020, A1 Location in patent:Paragraph 0345

2113-51-1
258 suppliers
$6.00/1g

829-85-6
280 suppliers
$33.00/25g

13885-09-1
186 suppliers
$6.00/250mg
![Phosphine oxide, [1,1'-biphenyl]-2-yldiphenyl-](/CAS/20200401/GIF/1942-82-1.gif)
1942-82-1
0 suppliers
inquiry

13885-09-1
186 suppliers
$6.00/250mg

2113-51-1
258 suppliers
$6.00/1g
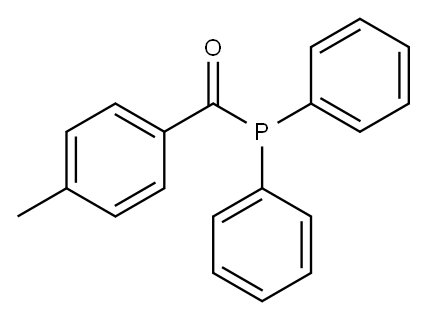
40841-62-1
0 suppliers
inquiry

13885-09-1
186 suppliers
$6.00/250mg
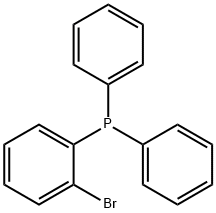
62336-24-7
121 suppliers
$24.00/250mg

98-80-6
743 suppliers
$5.00/5g

13885-09-1
186 suppliers
$6.00/250mg