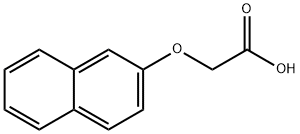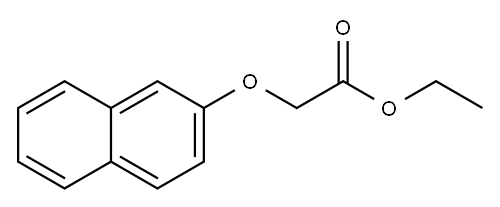
(2-naphthyloxy)acetic acid ethyl ester synthesis
- Product Name:(2-naphthyloxy)acetic acid ethyl ester
- CAS Number:6036-14-2
- Molecular formula:C14H14O3
- Molecular Weight:230.26
Yield:6036-14-2 95%
Reaction Conditions:
with potassium carbonate in acetone; for 4 h;Reflux;
Steps:
6.1.1. Ethyl 2-(naphthalen-2-yloxy) acetate (6a)
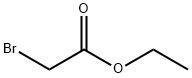
General procedure: A round-bottomed flask was charged with phenol or thiophenol compound (1.0 eq.), ethyl bromoacetate (1.2 eq.) and K2CO3 (3.0 eq.) followed by acetone (15 ml). The resulting mixture was refluxed in a pre-heated oil bath for 4 h under magnetic stirring. After cooling to room temperature, acetone was filtered over a pad of celite and rinsed with acetone (50 ml). The solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel with ethyl acetate/hexane (1:9) as eluent to afford corresponding esters (6a-7a & 15a-17a) in high purity with good yields.
References:
Sashidhara, Koneni V.;Palnati, Gopala Reddy;Dodda, Ranga Prasad;Sonkar, Ravi;Khanna;Bhatia, Gitika [European Journal of Medicinal Chemistry,2012,vol. 57,p. 302 - 310]
135-19-3
761 suppliers
$9.00/1g

6036-14-2
11 suppliers
inquiry