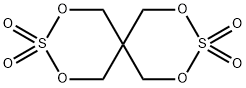
2,4,8,10-Tetraoxa-3,9-dithiaspiro[5.5]undecane, 3,3,9,9-tetraoxide synthesis
- Product Name:2,4,8,10-Tetraoxa-3,9-dithiaspiro[5.5]undecane, 3,3,9,9-tetraoxide
- CAS Number:201419-80-9
- Molecular formula:C5H8O8S2
- Molecular Weight:260.24
![2,4,8,10-Tetraoxa-3,9-disilaspiro[5.5]undecane, 3,3,9,9-tetramethyl-](/CAS/20210305/GIF/14896-25-4.gif)
14896-25-4
0 suppliers
inquiry
![2,4,8,10-Tetraoxa-3,9-dithiaspiro[5.5]undecane, 3,3,9,9-tetraoxide](/CAS/20200331/GIF/201419-80-9.gif)
201419-80-9
54 suppliers
inquiry
Yield:201419-80-9 92.53 %
Reaction Conditions:
with sulfuryl fluoride in 1,2-dichloro-ethane at 40 - 50; under 75.0075 Torr;Inert atmosphere;Green chemistry;Solvent;Temperature;Pressure;
Steps:
1; 4; 6 Example 4
1000mL three-port pressure-resistant flask, weigh 62.1 g (0.25mol) raw material 1 and 350g dichloroethane into a three-port flask, magnetic stirring,N2(10mL/min) replacement, the internal temperature of the control system is 40~50 °C, sulfonyl fluoride gas is slowly introduced to the system, the pressure of the system reaches 0.1Mpa, the air intake is stopped, the heat preservation reaction is 2.0h, and the nitrogen is vented. Continue to introduce sulfonyl fluoride gas to the system pressure of 0.1Mpa, heat preservation reaction 2.0h, nitrogen emptying, until GC tracking sampling, to confirm the completion of the reaction, stop the introduction of sulfonyl fluoride, the reaction into sulfonyl fluoride total 61.2g, the total reaction time is 7.0h.After confirming the completion of the reaction, filter under reduced pressure, add 300g of acetonitrile to the obtained filter cake, heat to 75~80 °C, the system is fully dissolved, cool down to 40~50 °C, add 5g of activated clay to the above system, and stir at 40~50 °C for 30min, suction, filter out the activated clay, and obtain colorless clear filtrate. The resulting colorless clear filtrate is desolved under reduced pressure and desolvated to the system with a slight solids precipitation.The internal temperature of the control system is 75-80 °C, the above system is heated to total dissolution, 500g of n-heptane is added to the system, a large amount of white solid is precipitated from the system, and the temperature is beaten and stirred for 30min, cooled to 0-5 °C, and the white solid is obtained by suction, and then dried under reduced pressure to obtain 60.2g of cyclic sulfate fine products, with a yield of 92.53%, GC purity of 99.94%, chromaticity 6Hazen (10% acetonitrile solution), acid value: 8ppm (in HF), Chloride content
References:
CN115368377,2022,A Location in patent:Paragraph 0022; 0041-0043; 0050-0052; 0064-0065
![2,4,8,10-tetraoxa-3,9-dithiaspiro[5.5]undecane 3,9-dioxide](/CAS/GIF/3670-93-7.gif)
3670-93-7
39 suppliers
inquiry
![2,4,8,10-Tetraoxa-3,9-dithiaspiro[5.5]undecane, 3,3,9,9-tetraoxide](/CAS/20200331/GIF/201419-80-9.gif)
201419-80-9
54 suppliers
inquiry
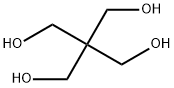
115-77-5
0 suppliers
$10.00/10g
![2,4,8,10-Tetraoxa-3,9-dithiaspiro[5.5]undecane, 3,3,9,9-tetraoxide](/CAS/20200331/GIF/201419-80-9.gif)
201419-80-9
54 suppliers
inquiry
