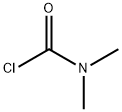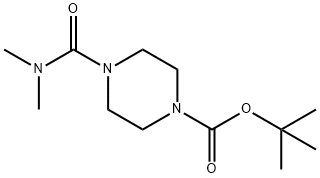
tert-Butyl 4-(dimethylcarbamoyl)piperazine-1-carboxylate, 1-(tert-Butoxycarbonyl)-4-(dimethylcarbamoyl)piperazine synthesis
- Product Name:tert-Butyl 4-(dimethylcarbamoyl)piperazine-1-carboxylate, 1-(tert-Butoxycarbonyl)-4-(dimethylcarbamoyl)piperazine
- CAS Number:215453-81-9
- Molecular formula:C12H23N3O3
- Molecular Weight:257.33
Yield:215453-81-9 97%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 12 h;
Steps:
8.2 Synthesis of tert-butyl 4-(dimeth lcarbamoyl)piperazine-l -carboxylate
To a stirred solution of tert-butyl piperazine-1 -carboxylate (step 1, 10.0 g, 53.76 mmol, 1.0 eq) in DCM (100 mL) was added dimethylcarbamic chloride (7.39 mL, 80.64 mmol, 1.5 eq) followed by TEA (11.18 mL, 80.64 mmol, 1.5 eq) at room temperature and stirred for about 12 hours at same temperature. TLC indicated starting material was consumed and the desired product was observed. The reaction mixture was diluted with water and extracted with DCM. The organic layer was washed with water, brine solution and was dried over Na2S04. Filtrate was concentrated to get the crude residue which was purified by column chromatography by using 2% MeOH in DCM as an eluent to obtain the desired product (13.5 g, yield: 97.0%) as a white solid.1H NMR (DMSO-d6, 300 MHz): δ 3.57-3.52 (m, 4H), 3.26-3.20 (m, 4H) and 1.07 (s, 6H) and 1.05 (s, 9H); Mass: [M+H-boc]+158.30 (100%).
References:
WO2017/149518,2017,A1 Location in patent:Page/Page column 35
506-59-2
556 suppliers
$5.00/5G
530-62-1
969 suppliers
$6.00/25g
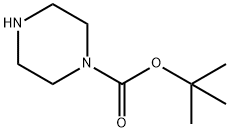
57260-71-6
729 suppliers
$5.00/5g

215453-81-9
27 suppliers
inquiry

124-40-3
545 suppliers
$18.00/100ml
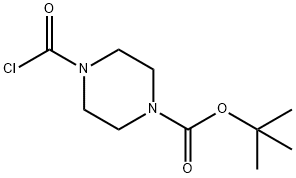
59878-28-3
56 suppliers
$98.00/100mg

215453-81-9
27 suppliers
inquiry

506-59-2
556 suppliers
$5.00/5G

59878-28-3
56 suppliers
$98.00/100mg

215453-81-9
27 suppliers
inquiry

24424-99-5
871 suppliers
$13.50/25G

215453-81-9
27 suppliers
inquiry