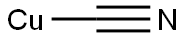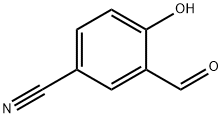
3-Formyl-4-hydroxybenzonitrile synthesis
- Product Name:3-Formyl-4-hydroxybenzonitrile
- CAS Number:74901-29-4
- Molecular formula:C8H5NO2
- Molecular Weight:147.13
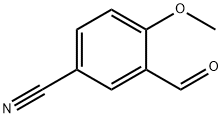
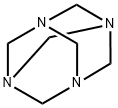
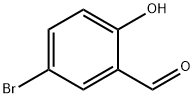
21962-53-8

74901-29-4
General procedure for the synthesis of 3-formyl-4-hydroxybenzonitrile from 5-cyano-2-methoxybenzaldehyde: B. Synthesis of 2-hydroxy-5-cyano benzaldehyde 31.08 g of 5-cyano-2-methoxybenzaldehyde obtained in the previous step and 24.52 g of lithium chloride were added to 500 mL of N,N-dimethylformamide. The reaction mixture was heated to reflux for 2 h. The solvent was subsequently removed by rotary evaporator. The residue was dissolved in aqueous acidic potassium sulfate solution and extracted with ethyl acetate. The organic phase was washed sequentially with water and saturated sodium chloride solution. After the above treatment, 24.5 g of the target product 2-hydroxy-5-cyanobenzaldehyde was obtained in 86% yield.

21962-53-8
37 suppliers
$48.39/250mg

74901-29-4
110 suppliers
inquiry
Yield:74901-29-4 86%
Reaction Conditions:
with lithium chloride in DMF (N,N-dimethyl-formamide)
Steps:
25.B
B. 2-Hydroxy-5-cyanobenzaldehyde 31.08 g of compound obtained in the preceding step and 24.52 g of lithium chloride are introduced into 500 ml of N,N-dimethylformamide. The mixture is brought to reflux for 2 hours and the solvent is evaporated off. The residue is taken up in a potassium acid sulfate solution and extraction is carried out with ethyl acetate. Washing is then carried out with water and a saturated sodium chloride solution. In this way, 24.5 g of desired compound are obtained. Yield: 86%
References:
Assens, Jean-Louis;Bernhart, Claude;Cabanel-Haudricourt, Frederique;Nisato, Dino US2004/10011, 2004, A1 Location in patent:Page/Page column 26
767-00-0
582 suppliers
$6.00/25g

141-78-6
683 suppliers
$20.50/250ml

74901-29-4
110 suppliers
inquiry

50-00-0
896 suppliers
$10.00/25g

767-00-0
582 suppliers
$6.00/25g

74901-29-4
110 suppliers
inquiry
161196-99-2
3 suppliers
inquiry