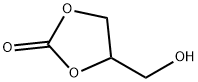
4-HYDROXYMETHYL-1,3-DIOXOLAN-2-ONE synthesis
- Product Name:4-HYDROXYMETHYL-1,3-DIOXOLAN-2-ONE
- CAS Number:931-40-8
- Molecular formula:C4H6O4
- Molecular Weight:118.09
Yield:931-40-8 100%
Reaction Conditions:
Candida antarctica lipase B in tert-butyl alcohol at 70; for 10 - 24 h;Enzyme kinetics;Enzymatic reaction;Molecular sieve;
Steps:
1; 2; 3
Based on the first estimation, mean values and a conversion ratio and yield with respect to each factor as shown in Table 2 below were measured in a case where the reaction temperature was about 70 °C, t-BuOH was used as the reaction solvent, and a molecular sieve (M.Sieve) was additionally contained in the reaction solvent. Similar to the first estimation, in order to produce a mean value of the combination of all factors for each level, a total of 27 data was produced. Meanwhile, an amount of the catalyst was determined to have a concentration of about 0.5 % or more based on the first estimation results.[Table 2]FIG. 4 is a graph showing a mean value of a conversion ratio with respect to changes in a catalyst concentration, a molecular sieve concentration, and a reaction time according to an exemplary embodiment of the invention, and FIG. 5 is a graph showing a mean value of a yield (%) with respect to changes in a catalyst concentration, a molecular sieve concentration, and a reaction time according to an exemplary embodiment of the invention. Referring to FIGS. 4 and 5, when the molecular sieve is used, both of the conversion ratio and yield increase. Also, the conversion ratio becomes nearly saturated for about 5 hours, however the yield continuously increases over time. Also, the conversion ratio and yield increase along with an increase in the concentration of the molecular sieve. FIG. 6 is a graph showing changes in a conversion ratio and yield over time with respect to all combinations of a catalyst concentration and molecular sieve
References:
GS CALTEX CORPORATION WO2009/35269, 2009, A2 Location in patent:Page/Page column title page; 6-9; 4/7 - 7/7

124-38-9
134 suppliers
$175.00/23402
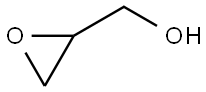
556-52-5
296 suppliers
$17.67/1gm:

931-40-8
194 suppliers
$8.00/5g
![1,3-Dioxolan-2-one, 4-[[(trimethylsilyl)oxy]methyl]-](/CAS/20210111/GIF/864079-61-8.gif)
864079-61-8
0 suppliers
inquiry

931-40-8
194 suppliers
$8.00/5g
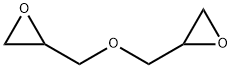
2238-07-5
39 suppliers
$47.75/50mg

124-38-9
134 suppliers
$175.00/23402

931-40-8
194 suppliers
$8.00/5g

