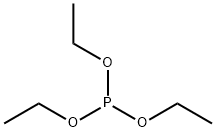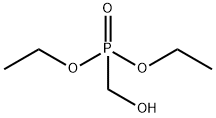
Diethyl (hydroxymethyl)phosphonate synthesis
- Product Name:Diethyl (hydroxymethyl)phosphonate
- CAS Number:3084-40-0
- Molecular formula:C5H13O4P
- Molecular Weight:168.13

50-00-0
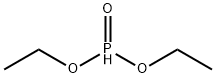
762-04-9

3084-40-0
1. In a 500 ml reaction flask, 76.6 g (0.554 moles) of diethyl phosphite, 20 g (0.667 moles added at 15%) of paraformaldehyde, 244 g (1.765 moles added at 15%) of potassium carbonate and 277 ml of toluene were added sequentially. The reaction mixture was heated to 60° C. After exothermic phenomena were observed, the remaining paraformaldehyde was added in batches and the addition process took about 15 minutes. 2. The reaction was continued at 60°C for 2 hours, during which time the remaining potassium carbonate was added in batches, maintaining the pH of the reaction system between 7.5 and 8. 3. Upon completion of the reaction, filtration was performed, and toluene was recovered by distillation under reduced pressure to obtain diethyl hydroxymethylphosphonate crude product of 92.1 g. The purity was detected by gas chromatography as ≥96%, and the yield was 95%. The product can be directly used in the next step of the reaction. 4. In a 1 liter reaction flask, 78.8 g (0.472 mol) of diethyl α-phosphonate, 278 mL of toluene and 90 g (0.889 mol) of triethylamine were added. Maintaining the temperature not lower than 30°C, 166 mL of toluene solution of toluene sulfonyl chloride 90 g (0.472 mol) was slowly added dropwise to the reaction vial, and the reaction was continued for 1 hour after the dropwise addition was completed. 5. The reaction system was warmed up to 60°C and the reaction was kept warm for 5 hours until high performance liquid chromatography (HPLC) showed that toluenesulfonyl chloride residue was ≤0.5%. 6. After the reaction was completed, the organic layer of toluene was washed twice with 277 ml of water, most of the toluene was removed by distillation under reduced pressure, a light yellow liquid was obtained, and 130.5 g of solid diethyl p-toluenesulfonyl oxymethoxyphosphonate was precipitated after cooling, with a yield of 87%.

50-00-0
896 suppliers
$10.00/25g

762-04-9
353 suppliers
$10.00/10 g

3084-40-0
256 suppliers
$10.00/10g
Yield:3084-40-0 96%
Reaction Conditions:
with triethylamine at 90; for 3 h;Reflux;
Steps:
O,O-Diethyl hydroxymethylphosphonate (10) [2]
Adapted from Phillion et al. To a stirred suspension of paraformaldehyde (2.22 g, 72 mmol, 1.00 equiv) in diethyl phosphite (11) (10.00 g, 72 mmol, 1.00 equiv) was added triethylamine (732 mg, 1.00 mL, 7 mmol, 0.10 equiv). The resulting suspension was heated to 90 °C and the suspension became clear and the mixture began reflux. The reaction was maintained at this temperature for three hours. The product was then purified by flash column chromatography (Rf 10 = 0.60, eluent - 100% EtOAC) and was isolated as a colourless oil (11.62 g, 70 mmol, 96%). 1H NMR (400 MHz, CDCl3): 1.35 (6H, t, J = 7.19 Hz, OCH2CH3), 3.91 (2H, dd appears as a triplet, JH-H = JH-P = 6.30 Hz, HOCH2P), 4.13-4.24 (4H, m, OCH2CH3), 4.81 - 4.86 (1H, m, HOCH2P). 13C NMR (100 MHz, CDCl3): 16.35 (d, J = 5.68 Hz, OCH2CH3), 56.87 (d, J = 162.21 Hz, HOCH2P), 62.51 (d, J = 6.76 Hz, OCH2CH3) 31P NMR (175 MHz, CDCl3): 24.52. HRMS (ESI+): Exact mass calc. C5H14O4P [M+H] = 169.0627; Found = 169.0630.
References:
Jones, David J.;O’Leary, Eileen M.;O’Sullivan, Timothy P. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 801 - 810] Location in patent:supporting information
![Phosphonic acid, [(acetyloxy)methyl]-, diethyl ester (9CI)](/CAS/20210111/GIF/7016-78-6.gif)
7016-78-6
0 suppliers
inquiry

3084-40-0
256 suppliers
$10.00/10g
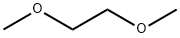
110-71-4
505 suppliers
$13.00/25ml

762-04-9
353 suppliers
$10.00/10 g

3084-40-0
256 suppliers
$10.00/10g
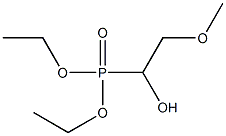
19462-41-0
2 suppliers
inquiry