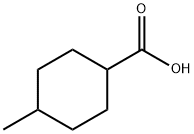
4-Methylcyclohexanecarboxylic acid synthesis
- Product Name:4-Methylcyclohexanecarboxylic acid
- CAS Number:4331-54-8
- Molecular formula:C8H14O2
- Molecular Weight:142.2
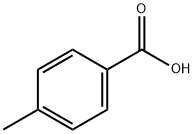
99-94-5
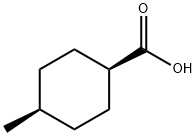
934-67-8

934-67-8
GENERAL STEPS: The hydrogenation reaction was carried out in a 25 mL stainless steel autoclave with a PTFE liner. In a typical experiment, 20 mg of catalyst and 61 mg (0.5 mmol) of p-toluic acid were dispersed in 5 mL of deionized water. After sealing the autoclave, the air in the autoclave was replaced with hydrogen and pressurized to 2.5 MPa. Subsequently, the reaction mixture was heated to 110 °C under magnetic stirring (1000 rpm). Upon completion of the reaction, it was cooled to room temperature, the reaction mixture was extracted with ethyl acetate and separated by centrifugation to remove the solid catalyst. The recovered solid catalyst was washed sequentially with ethyl acetate, ethanol and deionized water. The filtrate was analyzed by gas chromatography (GC, HP5890, USA) equipped with a 30 m HP-5 capillary column and a flame ionization detector (FID). All products were confirmed structurally by gas chromatography-mass spectrometry (GC-MS, Agilent 6890).
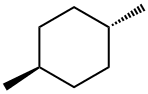
2207-04-7
38 suppliers
$92.00/5mL

4331-54-8
121 suppliers
$10.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 1.3 percent Chromat. / air (O2), AcOH, Zn /
2: 94 percent / pyridinium dichromate / dimethylformamide / 14 h / 20 °C
References:
Barton, Derek H. R.;Boivin, Jean;Gastiger, Michel;Morzycki, Jacek;Hay-Motherwell, Robyn S.;et al. [Journal of the Chemical Society. Perkin transactions I,1986,p. 947 - 956]

99-94-5
607 suppliers
$9.00/1g

4331-54-8
121 suppliers
$10.00/250mg
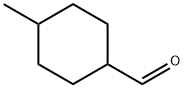
33242-79-4
12 suppliers
inquiry

4331-54-8
121 suppliers
$10.00/250mg
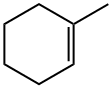
591-49-1
154 suppliers
$10.00/1g

124-38-9
133 suppliers
$214.00/14L

4331-54-8
121 suppliers
$10.00/250mg
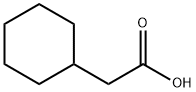
5292-21-7
205 suppliers
$9.00/1g
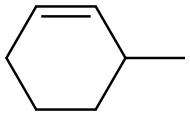
591-48-0
59 suppliers
$108.00/25mL

124-38-9
133 suppliers
$214.00/14L

4331-54-8
121 suppliers
$10.00/250mg

5292-21-7
205 suppliers
$9.00/1g