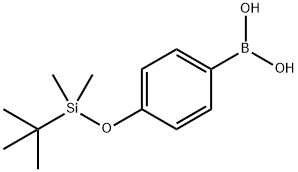
4-(TERT-BUTYLDIMETHYLSILYLOXY)PHENYLBORONIC ACID synthesis
- Product Name:4-(TERT-BUTYLDIMETHYLSILYLOXY)PHENYLBORONIC ACID
- CAS Number:159191-56-7
- Molecular formula:C12H21BO3Si
- Molecular Weight:252.19
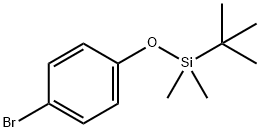
67963-68-2

159191-56-7
Step 1b: Synthesis of 4-(tert-butyldimethylsilyloxy)phenylboronic acid (compound 0102) To a solution of anhydrous THF (20 ml) of compound 0101 ((4-bromophenoxy)tert-butyldimethylsilane, 1.548 g, 5.389 mmol) was slowly added dropwise 2.5 M hexane solution of n-butyllithium (2.5 ml, 6.326 mmol) at -78 °C under N2 protection for 15 min. The reaction mixture was continued to be stirred at -78 °C for 0.5 h. Then trimethyl borate (730 mg, 7.029 mmol) was slowly added dropwise for 15 min. After the dropwise addition, the reaction mixture was continued stirring at -78°C for 1 hour, followed by slow warming to room temperature. The pH of the reaction mixture was adjusted to 5-7 with aqueous hydrochloric acid to quench the reaction. The solvent was removed under reduced pressure and the residue was extracted with dichloromethane (DCM). The organic layer was washed with saturated saline, dried over anhydrous sodium sulfate and concentrated to give the crude product. The crude product was washed with petroleum ether (2 ml) to give the target product 0102 as a white solid (1.102 g, 81% yield): LCMS: 253 [M + 1]+.
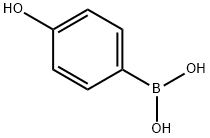
71597-85-8
334 suppliers
$6.00/1g

18162-48-6
677 suppliers
$9.00/5g

159191-56-7
121 suppliers
$20.00/250mg
Yield: 90%
Reaction Conditions:
with 1H-imidazole in N,N-dimethyl-formamide at 20; for 12 h;
Steps:
(4-((tert-Butyldimethylsilyl)oxy)phenyl)boronic Acid (9)
To a solution of 4-hydroxyphenylboronic acid (1.0 equiv., 5 mmol) in dry DMF (0.05 M) was added imidazole (5.0 equiv.) at rt, followed by the addition of TBSCl (3.5 equiv.). The reaction mixture was stirred at rt for 12 h; after completion monitored by TLC, the mixture was extracted with ethyl acetate (330 mL) and water (50 mL), and the organic layers were dried over MgSO4 and concentrated under vacuum. The residue was purified by flash chromatography (hexane/ethyl acetate 10 : 1 → 3 : 1) to give the product as a white solid (2.27 g, 90% yield). Rf (hexane/ethyl acetate 1 : 1) 0.30. δH (300 MHz, CDCl3) 8.11 (2H, d, J 8.4), 6.96 (2H, d, J 8.4), 1.02 (9H, s), 0.26 (6H, s). δC (75 MHz, CDCl3) 159.9, 137.6, 119.9, 111.0, 25.8, 18.4, 4.2. The spectroscopic data matched those reported in the literature.[15]
References:
Zhou, Qingqing;Reekie, Tristan A.;Abbassi, Ramzi H.;Venkata, Dinesh Indurthi;Font, Josep S.;Ryan, Renae M.;Rendina, Louis M.;Munoz, Lenka;Kassiou, Michael [Australian Journal of Chemistry,2018,vol. 71,# 10,p. 789 - 797]

67963-68-2
137 suppliers
$17.00/1g

159191-56-7
121 suppliers
$20.00/250mg

5419-55-6
388 suppliers
$12.00/5g

67963-68-2
137 suppliers
$17.00/1g

159191-56-7
121 suppliers
$20.00/250mg

18162-48-6
677 suppliers
$9.00/5g

159191-56-7
121 suppliers
$20.00/250mg

121-43-7
382 suppliers
$13.00/25mL

67963-68-2
137 suppliers
$17.00/1g

159191-56-7
121 suppliers
$20.00/250mg