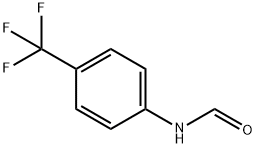
4-(Trifluoromethyl)formanilide synthesis
- Product Name:4-(Trifluoromethyl)formanilide
- CAS Number:74702-40-2
- Molecular formula:C8H6F3NO
- Molecular Weight:189.13
455-14-1
522 suppliers
$5.00/10g
201230-82-2
1 suppliers
inquiry

74702-40-2
19 suppliers
$45.00/10mg
Yield:74702-40-2 96 %Chromat.
Reaction Conditions:
with sodium tertiary butoxide in N,N-dimethyl acetamide at 100; under 1500.15 Torr; for 12 h;Autoclave;Pressure;
Steps:
45; 46 The preparation of p-trifluoromethylformanilide B19 is shown in schematic diagram 27 of its synthetic route.
In a glove box, add p-trifluoromethylaniline (10.0 mmol), tBuONa (10.0 mmol) and DMAc (10.0 mL) to a dry reaction vial with a magnetic stir bar,The reaction vial was placed in an autoclave, and after the CO gas was replaced three times in the autoclave, CO (2 bar) was charged. After the reaction was uniformly stirred at room temperature, the autoclave was placed in a preheated oil bath, and the reaction was carried out at 100 °C for 12 h. The reaction was stopped, and the column chromatography separation yield was 96%.
References:
CN115043750,2022,A Location in patent:Paragraph 0246-0261

455-14-1
522 suppliers
$5.00/10g

124-38-9
134 suppliers
$214.00/14L

74702-40-2
19 suppliers
$45.00/10mg
![1H-Imidazole-1-carboxamide, N-[4-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/1515782-30-5.gif)
1515782-30-5
0 suppliers
inquiry

74702-40-2
19 suppliers
$45.00/10mg

64-18-6
1132 suppliers
$22.12/250ML

455-14-1
522 suppliers
$5.00/10g

74702-40-2
19 suppliers
$45.00/10mg

455-14-1
522 suppliers
$5.00/10g

66-25-1
335 suppliers
$16.80/100G

74702-40-2
19 suppliers
$45.00/10mg