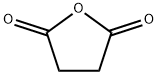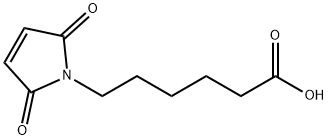
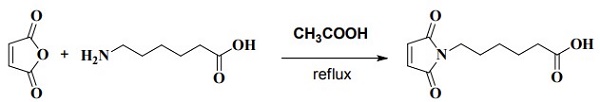
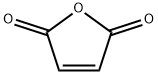
6-Maleimidocaproic acid synthesis
- Product Name:6-Maleimidocaproic acid
- CAS Number:55750-53-3
- Molecular formula:C10H13NO4
- Molecular Weight:211.21
Yield:55750-53-3 77.4%
Reaction Conditions:
with acetic acid at 20; for 10 h;Reflux;Time;
Steps:
A
Compound 1 (150 g, 1.53 mol) was added to a stirred solution of Compound 2 (201 g, 1.53 mol) in HOAc (1000 mL). After the mixture was stirred at r.t. for 2 h, it was heated at reflux for 8 h. The organic solvents were removed under reduced pressure and the residue was extracted with EtOAc (500 mL x 3), washed with H20. The combined organic layers was dried over Na2S04 and concentrated to give the crude product. It was washed with petroleum ether to give compound 3 as white solid (250 g, 77.4 %).
References:
GENENTECH, INC.;THE UNIVERSITY OF AUCKLAND;LEE, Ho Huat;TERCEL, Moana;FLYGARE, John A.;GUNZNER-TOSTE, Janet;PILLOW, Thomas H.;SAFINA, Brian;STABEN, Leanna;VERMA, Vishal;WEI, BinQing;ZHAO, Guiling WO2015/95227, 2015, A2 Location in patent:Page/Page column 261; 262
108-31-6
886 suppliers
$13.47/50 G
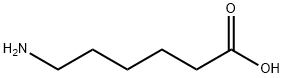
60-32-2
611 suppliers
$5.00/10g

55750-53-3
328 suppliers
$9.00/1g
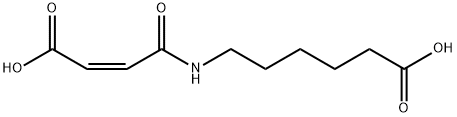
57079-14-8
29 suppliers
inquiry

55750-53-3
328 suppliers
$9.00/1g

57079-14-8
29 suppliers
inquiry

55750-53-3
328 suppliers
$9.00/1g

108-31-6
886 suppliers
$13.47/50 G
7440-44-0
352 suppliers
$12.00/25g

60-32-2
611 suppliers
$5.00/10g

55750-53-3
328 suppliers
$9.00/1g