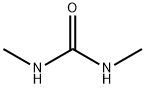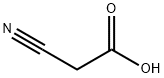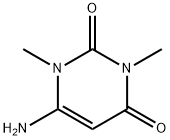
6-Amino-1,3-dimethyl-1,2,3,4-tetrahydropyrimidine-2,4-dione synthesis
- Product Name:6-Amino-1,3-dimethyl-1,2,3,4-tetrahydropyrimidine-2,4-dione
- CAS Number:6642-31-5
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15
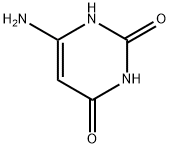
873-83-6

74-88-4

6642-31-5
General procedure for the synthesis of 1,3-dimethyl-6-aminouracil from 4-amino-2,6-dihydroxypyrimidine and iodomethane: 4-amino-2,6-dihydroxypyrimidine (1.27 g, 10 mmol) was added to a pre-heated ethanol solution (50 ml) containing KOH (0.56 g, 10 mmol) and heated continuously for 40 min. After completion of the reaction, the mixture was cooled to room temperature followed by addition of iodomethane (20 mmol). The reaction mixture was stirred under heating conditions for 8 hours, after which it was cooled to room temperature. The reaction mixture was poured into cold distilled water (100 ml) to induce precipitation of the final product 1,3-dimethyl-6-aminouracil. The precipitate was collected by filtration and washed with distilled water (100 ml). Finally, recrystallization from methanol gave 1,3-dimethyl-6-aminouracil in yellow crystalline form.
Yield:6642-31-5 90%
Reaction Conditions:
Stage #1:4-Amino-2,6-dihydroxypyrimidine with potassium hydroxide in ethanol for 0.666667 h;Heating;
Stage #2:methyl iodide in ethanol for 8 h;Heating;
Steps:
6-Amino-1, 3-dimethyl-1H-pyrimidine-2, 4-Dione (2)
To a heated ethanolic KOH solution (0.56 g, 10 mmol in 50 ml ethanol) compound 1 (1.27 g, 10 mmol) was added and heating was continuous for 40 min. The mixture was allowed to cool to room temperature, and methyl iodide (20 mmol) was added. The mixture was stirred under heating for 8 h and then allowed to cool to room temperature and finally poured into cold H2O (100 ml), The final product ppt. was filtered and washed with 100 ml H2O and crystallized from methanol to afford 2 as yellow crystals.
References:
Abu-Hashem, Ameen Ali;Hussein, Hoda Abdel Raouf [Letters in drug design and discovery,2015,vol. 12,# 6,p. 471 - 478]
![2-cyano-N-methyl-N-[(methylamino)carbonyl]acetamide](/CAS/GIF/39615-79-7.gif)
39615-79-7
42 suppliers
inquiry

6642-31-5
398 suppliers
$5.00/10g
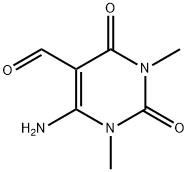
54660-80-9
31 suppliers
$144.00/1ea
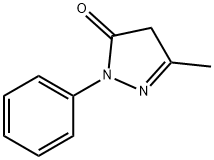
89-25-8
855 suppliers
$6.00/25g

6642-31-5
398 suppliers
$5.00/10g
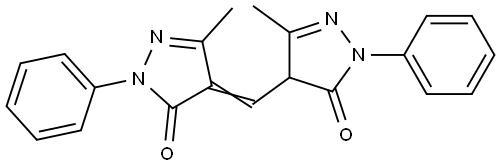
4702-90-3
168 suppliers
inquiry