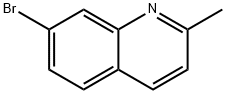
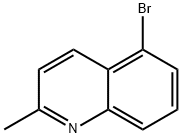
7-BROMO-2-METHYLQUINOLINE synthesis
- Product Name:7-BROMO-2-METHYLQUINOLINE
- CAS Number:4965-34-8
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
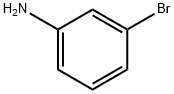
591-19-5
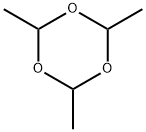
123-63-7

4965-34-8
Doebner-Miller Synthesis: 3-Bromoaniline (10 mL, 92 mmol) was slowly added to a 37% hydrochloric acid solution (200 mL) pre-cooled to 0°C. Subsequently, trimeric acetaldehyde (11 mL, 0.8 mol, 9 equiv) was added dropwise to the reaction system. The reaction mixture was stirred at room temperature for 1 hour and then heated to reflux temperature to continue the reaction for 3 hours. Upon completion of the reaction, the mixture was cooled to 0 °C and saturated aqueous sodium hydroxide solution (200 mL) was slowly added to neutralize the acidity. The reaction mixture was extracted with dichloromethane (200 mL x 3) and the organic phases were combined. The organic phase was washed with water and saturated saline in turn, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to obtain the crude product. The crude product was a mixture of 5-bromoquinolidine and 7-bromoquinolidine, which was separated and purified by column chromatography (silica gel, cyclohexane-ethyl acetate=9:1) to obtain the 7-bromoquinolidine regiosemide as a light yellow solid (9.3 g, 46% yield). Molecular formula: C10H8BrN. molecular weight: 222.08 g/mol. IR (thin film method): 1610, 1494, 1264, 841, 736 cm-1. melting point: 57°C. 1H NMR (CDCl3): δ 8.09 (s, 1H, H8), 7.80 (d, J = 8.2 Hz, 1H, H4), 7.39 (m, 2H, H5 and H7). 2H, H5 and H7), 7.12 (d, J = 8.2 Hz, 1H, H3), 2.61 (s, 3H, H9).13C NMR (CDCl3): δ 160.3 (C2), 148.6 (C8a), 136.2 (C4), 131.2 (C8), 129.4 (C5), 128.9 (C6), 125.3 ( C4a), 123.7 (C7), 122.6 (C3), 25.7 (C9).

591-19-5
334 suppliers
$10.00/10g

123-63-7
259 suppliers
$30.50/8182550100

4965-34-8
80 suppliers
$65.00/50mg
Yield: 46%
Reaction Conditions:
Stage #1:m-Bromoaniline;paracetaldehyde with hydrogenchloride in water at 0 - 20; for 4 h;Reflux;Doebner-Miller Synthesis;
Stage #2: with sodium hydroxide in water at 0;
Steps:
Doebner Miller synthesis: 3-Bromoaniline (10 mL, 92 mmol) was added to a solution of 37 % HCl at 0 °C (200 mL). Paraldehyde (11 mL, 0.8 mol, 9 eq) was then introduced and the mixture was left to react at room temperature for 1 hour, and then heated to reflux temperature for 3 hours. After cooling to 0 °C, a saturated aquous solution of sodium hydroxide (200 mL) was slowly added and the mixture was extracted with dichloromethane. The organic layer was washed with water and brine, then dried over MgSO4, and concentrated under reduced pressure. The crude product was obtained as a mixture of 5-bromoquinaldine and 7-bromoquinaldine that were separated by column chromatography (SiO2, cyclohexane-AcOEt 9:1). The 7-bromoquinaldine regioisomer was obtained as a sand yellow solid (9,3 g, 46 %). Molecular formula: C10H8BrN. Molecular weight: 222.08 g.mol-1. IR (film): 1610, 1494, 1264, 841, 736 cm-1. Tfusion: 57 °C. 1H NMR: δ 8.09 (s, 1H, H8), 7.80 (d, J = 8.2 Hz, 1H, H4), 7.39 (m, 2H, H5 et H7), 7.12 (d, J = 8.2 Hz, 1H, H3), 2.61 (s, 3H, H9). 13C NMR: δ 160.3 (s, C2), 148.6 (s, C8a), 136.2 (s, C4), 131.2 (s, C8), 129.4 (s, C5), 128.9 (s, C6), 125.3 (s, C4a), 123.7 (s, C7), 122.6 (s, C3), 25.7 (s, C9).
References:
Centre National de la Recherche Scientifique CNRS;Université Paris Descartes EP2397466, 2011, A1 Location in patent:Page/Page column 12
50-00-0
896 suppliers
$10.00/25g

591-19-5
334 suppliers
$10.00/10g

4965-34-8
80 suppliers
$65.00/50mg

591-19-5
334 suppliers
$10.00/10g

123-63-7
259 suppliers
$30.50/8182550100

4965-34-8
80 suppliers
$65.00/50mg
54408-52-5
60 suppliers
inquiry

123-73-9
235 suppliers
$40.00/5g

591-19-5
334 suppliers
$10.00/10g

4965-34-8
80 suppliers
$65.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4965-34-8
80 suppliers
$65.00/50mg