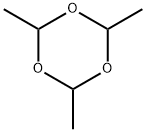
Paraldehyde synthesis
- Product Name:Paraldehyde
- CAS Number:123-63-7
- Molecular formula:C6H12O3
- Molecular Weight:132.16

64-17-5
826 suppliers
$10.00/50g

75-07-0
429 suppliers
$14.00/5mL
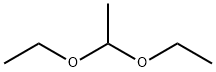
105-57-7
315 suppliers
$18.00/25mL

6117-91-5
156 suppliers
$46.50/25ml
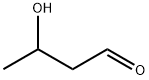
107-89-1
33 suppliers
$335.00/50g

141-78-6
685 suppliers
$20.50/250ml

123-63-7
257 suppliers
$30.50/8182550100

123-73-9
233 suppliers
$40.00/5g
Yield:-
Reaction Conditions:
with CO2-loaded faujasite zeolite NaY at 230; under 15514.9 Torr; for 6 h;Inert atmosphere;Autoclave;Reagent/catalyst;
Steps:
2.3. Catalytic reaction measurements
General procedure: The faujasite-catalyzed aldol condensation of acetaldehyde was carried out in the liquid-phase using a high-pressure 50-mL stainless steel autoclave batch reactor (Parr Corporation) equipped with impeller, temperature and pressure controllers, and sampling port. Ethanol was selected as the solvent to simulate conditions that might be encountered in the ethanol-to-BD process. In such a pro-cess, only a fraction of ethanol is dehydrogenated to acetaldehyde,which undergoes the aldol condensation; the unconverted ethanol can be used as a hydrogen source in the MPV reaction that converts crotonaldehyde to crotyl alcohol. Reaction temperature range was 130-230°C, while the total pressure was kept at 300 psi. In a typical experiment, a measured amount of catalyst was mixed with acetaldehyde and ethanol in the reactor vessel. After the reactor was sealed, it was purged with N2 to 300 psi, and heated up to the desired reaction temperature while stirring at 300-400 rpm. In all runs, the initial ethanol:acetaldehyde volume ratio was kept at 10:1(2 mL of acetaldehyde). After a given period of time (6 or 12 h), the reaction was quenched by rapid cooling. Liquid products were filtered and analyzed using gas chromatography, GC-MS for product identification and GC-FID for quantification. Chemical standards were used to obtain the retention times and response factors for all reactants and products.
References:
Zhang, Lu;Pham, Tu N.;Faria, Jimmy;Resasco, Daniel E. [Applied Catalysis A: General,2015,vol. 504,p. 119 - 129]

75-07-0
429 suppliers
$14.00/5mL

123-63-7
257 suppliers
$30.50/8182550100

123-73-9
233 suppliers
$40.00/5g

75-07-0
429 suppliers
$14.00/5mL

123-63-7
257 suppliers
$30.50/8182550100
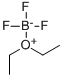
109-63-7
403 suppliers
$10.60/1gm:

75-07-0
429 suppliers
$14.00/5mL

123-63-7
257 suppliers
$30.50/8182550100

75-07-0
429 suppliers
$14.00/5mL
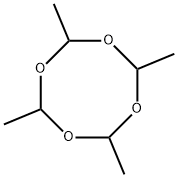
108-62-3
241 suppliers
$17.00/25g

123-63-7
257 suppliers
$30.50/8182550100