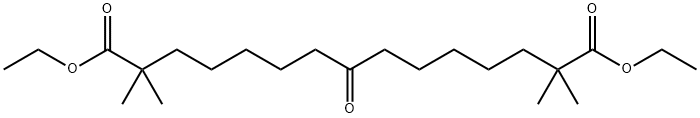
2,2,14,14-Tetramethyl-8-oxopentadecanedioic acid diethyl ester synthesis
- Product Name:2,2,14,14-Tetramethyl-8-oxopentadecanedioic acid diethyl ester
- CAS Number:738606-43-4
- Molecular formula:C23H42O5
- Molecular Weight:398.58
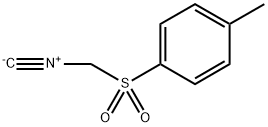
36635-61-7
522 suppliers
$5.00/5g
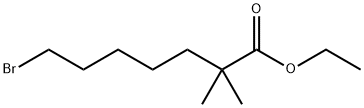
123469-92-1
151 suppliers
inquiry

738606-43-4
122 suppliers
inquiry
Yield:738606-43-4 53%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;sodium hydride in dimethyl sulfoxide at 20; for 12 h;Inert atmosphere;
Steps:
1.2 Step 2 Synthesis of Compound 5
Under 0 °C nitrogen protection conditions,Sodium hydride (0.3 g, 12.4 mmol) was slowly added to compound 3 (3.0 g, 11.3 mmol),a solution of tetrabutyl iodide (0.42 g, 1.13 mmol) and compound 4 (1.1 g, 5.63 mmol) in DMSO (20 mL)After the addition, the reaction was carried out at room temperature for 12 h.The reaction was quenched by the addition of ice water (40 ml).Extracted with dichloromethane (50 ml × 3), combined organic phase,Dry over anhydrous sodium sulfate and concentrate to about 30 ml of solvent.Concentrated hydrochloric acid (15 ml) was added and stirred at room temperature for 2 h.Then separate the organic phase,Wash with sodium bicarbonate solution, remove the solvent,The concentrate was subjected to column separation (eluent: petroleum ether: ethyl acetate = 20:1).1.2 g of a colorless liquid was obtained in a yield of 53%.
References:
CN110054562,2019,A Location in patent:Paragraph 0161; 0164; 0167; 0168
![Pentadecanedioic acid, 8-isocyano-2,2,14,14-tetramethyl-8-[(4-methylphenyl)sulfonyl]-, 1,15-diethyl ester](/CAS/20200401/GIF/738606-44-5.gif)
738606-44-5
21 suppliers
inquiry

738606-43-4
122 suppliers
inquiry

111-24-0
407 suppliers
$5.00/5g

738606-43-4
122 suppliers
inquiry
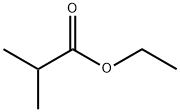
97-62-1
279 suppliers
$16.60/100G

738606-43-4
122 suppliers
inquiry

123469-92-1
151 suppliers
inquiry

738606-43-4
122 suppliers
inquiry