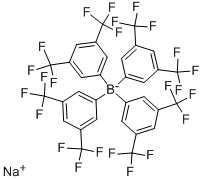
Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate synthesis
- Product Name:Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
- CAS Number:79060-88-1
- Molecular formula:C32H12BF24Na
- Molecular Weight:886.2
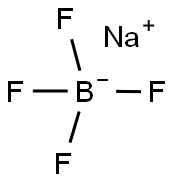
13755-29-8
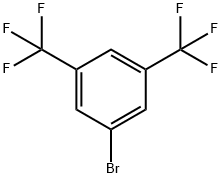
328-70-1
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/CAS/GIF/79060-88-1.gif)
79060-88-1
The general procedure for the synthesis of sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate from sodium fluoborate and 3,5-bis(trifluoromethyl)bromobenzene was as follows: first, a three-necked round-bottomed flask fitted with a reflux condenser was evacuated, flame-dried and replaced with argon. Subsequently, 1.01 g (41.7 mmol) of magnesium shavings, 0.72 g (6.4 mmol, 1 equiv.) of sodium fluoborate (NaBF4) and 150 mL of anhydrous ether were added to the flask. To initiate the reaction, 1.07 g (0.49 mL, 5.7 mmol, 0.9 eq.) of dibromoethane was added, and after heating the flask slightly for a few minutes, 1.71 g (6.25 mL, 36 mmol) of 3,5-bis(trifluoromethyl)bromobenzene was slowly added dropwise. After 30 minutes of reaction, the reaction mixture was diluted with 50 mL of ether. After the exothermic reaction slowed down, the reaction mixture was continued to be heated for 30 minutes. Subsequently, the reaction solution was stirred at room temperature overnight. Upon completion of the reaction, 16 g of sodium carbonate (Na2CO3) was added to 200 mL of distilled water to quench the reaction, stirred for 30 minutes and then filtered. The aqueous phase was extracted three times with 50 mL of ether, the organic phases were combined and dried with anhydrous sodium sulfate and activated carbon, and filtered. The solvent was removed under reduced pressure and the residual crude product was dissolved in 200 mL of toluene and the water was removed by azeotropic boiling through a Dean-Stark water separator. The solvent was again removed under reduced pressure, the product was filtered, washed with anhydrous toluene and dried under vacuum to give 4.65 g (5.3 mmol, 82% yield) of a colorless solid product with melting point decomposition >290 °C. The product was subjected to 1H-NMR (300 MHz, DMSO-d6), 13C-NMR (75.5 MHz, DMSO-d6), 19F-NMR (283 MHz, DMSO-d6) and elemental analysis to confirm the structure. Elemental analysis (C32H12BF24Na-1.8H2O) Calculated value: C=41.61, H=1.70; measured value: C=41.57, H=1.66.
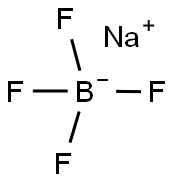
13755-29-8
495 suppliers
$6.00/25g

328-70-1
438 suppliers
$10.00/25 g
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/CAS/GIF/79060-88-1.gif)
79060-88-1
152 suppliers
$9.00/250mg
Yield:79060-88-1 82%
Reaction Conditions:
with magnesium;ethylene dibromide in diethyl ether at 20;Inert atmosphere;Heating;
Steps:
Synthesis of sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate NaB[3,5-(CF3)2C6H3]4
A three-neck round bottom flask fitted with a reflux condenser was evacuated, flame dried and filledwith argon prior to use. 1.01 g (41.7 mmol) magnesium, 0.72 g (6.4 mmol, 1 eq) NaBF4 and diethylether (150 mL) were added. To start to reaction 1.07 g (0.49 ml, 5.7 mmol, 0.9 eq) dibromoethanewere added and the flask was heated for several minutes followed by the dropwise addition of 1.71 g(6.25 ml, 36 mmol) 3,5-bis(trifluoromethyl)bromobenzene diluted with diethyl ether (50 mL) over30 min. When the exothermic reaction slowed the reaction mixture was heated for additional 30 min.The solution was then stirred over night at room temperature. The reaction mixture was quenched bythe addition of 16 g Na2CO3 in distilled water (200 mL), stirred for 30 min and filtered. The aqueousphase was extracted three times with diethyl ether (50 mL), the combined organic phases were driedover sodium sulfate and charcoal followed by filtration. The solvent was removed and the remainingcrude product was dissolved in toluene (200 mL) to remove the water with a Dean Stark trap byazeotropic distillation. The solvent was removed, the product filtered, washed with dry toluene anddried under vacuo. The product was observed as colorless solid (4.65 g, 5.3 mmol, 82 %).m.p. decomposition >290 °C.1H-NMR (300 MHz, DMSO-d6): δ = 7.67 (s, 4H, B-p-CH), 7.61 (s, 8H, B-o-CH) ppm.13C-NMR (75.5 MHz, DMSO-d6): δ = 161.0 (q, JB = 50 Hz, 4 Ci-B), 134.0 (s, 8 B-o-CH), 128.5 (qq,JF = 31 Hz, JB = 2.7 Hz, 8 Ci-CF3), 124.0 (q, JF = 272 Hz, CF3), 117.6 (m, 4 B-p-CH) ppm.19F-NMR (283 MHz, DMSO-d6): δ = -57.6 (CF3) ppm.Elemental analysis for C32H12BF24Na*1.8 H2O: calcd. C = 41.61 H = 1.70, found C = 41.57, H = 1.66.
References:
Kaliner, Maria;Strassner, Thomas [Tetrahedron Letters,2016,vol. 57,# 31,p. 3453 - 3456] Location in patent:supporting information

328-70-1
438 suppliers
$10.00/25 g
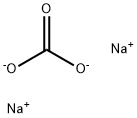
497-19-8
1488 suppliers
$13.00/300G
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/CAS/GIF/79060-88-1.gif)
79060-88-1
152 suppliers
$9.00/250mg

328-70-1
438 suppliers
$10.00/25 g
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/CAS/GIF/79060-88-1.gif)
79060-88-1
152 suppliers
$9.00/250mg

328-70-1
438 suppliers
$10.00/25 g
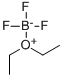
109-63-7
407 suppliers
$10.60/1gm:

497-19-8
1488 suppliers
$13.00/300G
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/CAS/GIF/79060-88-1.gif)
79060-88-1
152 suppliers
$9.00/250mg