Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
- CAS No.
- 79060-88-1
- Chemical Name:
- Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
- Synonyms
- NABARF;TFPB;NaBARF Hydrate;Kobayashi's Reagent;Na[B(3,5-(CF3)2C6H3)4];Tfpb(Kobayashi'SReagent);Sodium tetrakis[3,5-bis(trifL;Sodium tetrakis£3,5-bis(triflu;Tetra (3,5-difluoromethyl) phenyl) sodium borate;Benzene, 1,3-bis(trifluoromethyl)-, boron complex
- CBNumber:
- CB0311759
- Molecular Formula:
- C32H12BF24Na
- Molecular Weight:
- 886.2
- MDL Number:
- MFCD00043323
- MOL File:
- 79060-88-1.mol
- MSDS File:
- SDS
| Melting point | 310℃ |
|---|---|
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly) |
| form | Powder |
| color | tan |
| Water Solubility | soluble |
| BRN | 5474788 |
| InChIKey | LTGMONZOZHXAHO-UHFFFAOYSA-N |
| SMILES | [B-](C1=CC(=CC(C(F)(F)F)=C1)C(F)(F)F)(C1=CC(=CC(C(F)(F)F)=C1)C(F)(F)F)(C1=CC(=CC(C(F)(F)F)=C1)C(F)(F)F)C1C=C(C=C(C(F)(F)F)C=1)C(F)(F)F.[Na+] |
| CAS DataBase Reference | 79060-88-1 |
| UNSPSC Code | 26111700 |
| NACRES | NB.61 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 36/37/38-20/21/22 | |||||||||
| Safety Statements | 36/37/39-26-22 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10 | |||||||||
| TSCA | No | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 692360 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate | 79060-88-1 | 250mg | $186 | 2025-07-31 | Buy |
| Sigma-Aldrich | 692360 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate | 79060-88-1 | 1g | $361 | 2025-07-31 | Buy |
| TCI Chemical | S0447 | Sodium Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Hydrate >98.0%(HPLC)(W) | 79060-88-1 | 200mg | $42 | 2025-07-31 | Buy |
| TCI Chemical | S0447 | Sodium Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Hydrate >98.0%(HPLC)(W) | 79060-88-1 | 1g | $163 | 2025-07-31 | Buy |
| Strem Chemicals | 11-0590 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, min. 98% NaBARF | 79060-88-1 | 50mg | $85 | 2024-03-01 | Buy |
Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Chemical Properties,Uses,Production
Chemical Properties
Light beige powder
Uses
Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate was used in the preparation of poly(n-butyl acrylate)- based ion-selective membranes.
Uses
Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, also known as the BARF anion, is a compound used in catalytic cycles due to its inability to coordinate to metal centres.
General Description
Visit our Sensor Applications portal to learn more.
reaction suitability
core: sodium
reagent type: catalyst
Synthesis
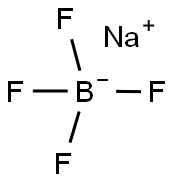
13755-29-8
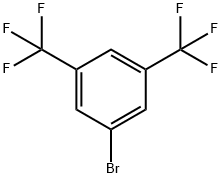
328-70-1
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/CAS/GIF/79060-88-1.gif)
79060-88-1
The general procedure for the synthesis of sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate from sodium fluoborate and 3,5-bis(trifluoromethyl)bromobenzene was as follows: first, a three-necked round-bottomed flask fitted with a reflux condenser was evacuated, flame-dried and replaced with argon. Subsequently, 1.01 g (41.7 mmol) of magnesium shavings, 0.72 g (6.4 mmol, 1 equiv.) of sodium fluoborate (NaBF4) and 150 mL of anhydrous ether were added to the flask. To initiate the reaction, 1.07 g (0.49 mL, 5.7 mmol, 0.9 eq.) of dibromoethane was added, and after heating the flask slightly for a few minutes, 1.71 g (6.25 mL, 36 mmol) of 3,5-bis(trifluoromethyl)bromobenzene was slowly added dropwise. After 30 minutes of reaction, the reaction mixture was diluted with 50 mL of ether. After the exothermic reaction slowed down, the reaction mixture was continued to be heated for 30 minutes. Subsequently, the reaction solution was stirred at room temperature overnight. Upon completion of the reaction, 16 g of sodium carbonate (Na2CO3) was added to 200 mL of distilled water to quench the reaction, stirred for 30 minutes and then filtered. The aqueous phase was extracted three times with 50 mL of ether, the organic phases were combined and dried with anhydrous sodium sulfate and activated carbon, and filtered. The solvent was removed under reduced pressure and the residual crude product was dissolved in 200 mL of toluene and the water was removed by azeotropic boiling through a Dean-Stark water separator. The solvent was again removed under reduced pressure, the product was filtered, washed with anhydrous toluene and dried under vacuum to give 4.65 g (5.3 mmol, 82% yield) of a colorless solid product with melting point decomposition >290 °C. The product was subjected to 1H-NMR (300 MHz, DMSO-d6), 13C-NMR (75.5 MHz, DMSO-d6), 19F-NMR (283 MHz, DMSO-d6) and elemental analysis to confirm the structure. Elemental analysis (C32H12BF24Na-1.8H2O) Calculated value: C=41.61, H=1.70; measured value: C=41.57, H=1.66.
References
[1] Inorganic Chemistry, 2014, vol. 54, # 1, p. 359 - 369
[2] Inorganic Chemistry, 2014, vol. 54, # 1, p. 359 - 369
[3] Tetrahedron Letters, 2016, vol. 57, # 31, p. 3453 - 3456
[4] Organic Syntheses, 2008, vol. 85, p. 248 - 266
[5] Journal of the American Chemical Society, 2001, vol. 123, # 44, p. 11020 - 11028
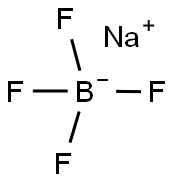

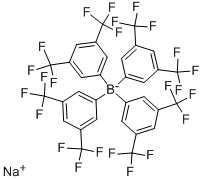
Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20202 | 58 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1803 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | FandaChem@Gmail.com | China | 1631 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29855 | 58 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 21193 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49733 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9266 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | info@dycnchem.com | China | 52846 | 58 |
| Henan Alfa Chemical Co., Ltd | +8615838112936 | alfa10@alfachem.cn | China | 13014 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
View Lastest Price from Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate pictures](/ProductImageEN/2024-03/Small/c8ad6dd0-09cb-47e4-a8e0-bca2683c5a62.gif) |
2024-03-26 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
79060-88-1
|
US $5.00-0.20 / KG | 1KG | 98% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate pictures](/ProductImageEN/2023-03/Small/4fb62407-afdf-462a-9de3-444c957afd89.png) |
2023-03-11 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
79060-88-1
|
US $0.00 / kg | 1kg | 95.0%min | 1000kg | LY Global chemicals co.,ltd . | |
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate pictures](/ProductImageEN/2021-08/Small/c2425313-e006-4218-b93e-70320eac1ee0.jpg) |
2021-08-12 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
79060-88-1
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate pictures](/ProductImageEN/2024-03/Small/c8ad6dd0-09cb-47e4-a8e0-bca2683c5a62.gif)
- Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
79060-88-1
- US $5.00-0.20 / KG
- 98%
- Henan Fengda Chemical Co., Ltd
-
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate pictures](/ProductImageEN/2023-03/Small/4fb62407-afdf-462a-9de3-444c957afd89.png)
- Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
79060-88-1
- US $0.00 / kg
- 95.0%min
- LY Global chemicals co.,ltd .
-
![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate pictures](/ProductImageEN/2021-08/Small/c2425313-e006-4218-b93e-70320eac1ee0.jpg)
- Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
79060-88-1
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
79060-88-1(Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate)Related Search:
1of4





![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Structure](CAS/GIF/79060-88-1.gif)