
AKOS BBS-00005410 synthesis
- Product Name:AKOS BBS-00005410
- CAS Number:38002-61-8
- Molecular formula:C14H10ClNO
- Molecular Weight:243.69
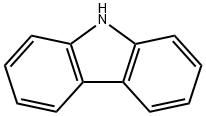
86-74-8
347 suppliers
$10.00/5g
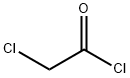
79-04-9
382 suppliers
$12.00/5g

38002-61-8
16 suppliers
$57.50/250mg
Yield:38002-61-8 90%
Reaction Conditions:
in N,N-dimethyl-formamide at 80; for 48 h;
Steps:
5.1.2. 10-(Chloroacetyl)-10H-carbazole 12
To a solution of carbazole (400 mg, 2.39 mmol) in dry DMF (4 mL) was added drop by drop α-chloro acetyl chloride (0.95 ml). The reaction was maintaining to 80 °C for 2 d. The solvent was remove in vacuo and the reaction mixture was treated with a solution of NaOH 5% and extracted with dichloromethane (3 × 10 mL). Then the organic layer was dried over anhydrous Na2SO4. The solvent was removed in vacuo and the crude product was purified by p-TLC using as eluent hexane:dichloromethane (1:1 v/v). Compound 12 was obtained as a white solid. Mp: 88-92 °C, yield 90%. IR (KBr, cm-1): 1699 (CO). 1H-NMR (500 MHz, CDCl3): δ 4.72 (s, 2H, CH2CI), 7.45 (t, J = 10 Hz, 2H, Ar), 7.55 (t, J = 10 Hz, 2H, Ar), 8.03 (d, J = 10 Hz, 2H, Ar), 8.2 (d, J = 10 Hz, 2H, Ar) ppm. 13C-NMR (75 MHz, CDCl3): δ 44.9, 116.2, 120.0, 124.4, 126.7, 127.6, 137.9, 165.6 ppm. HRMS (ESI): calcd for C14H11ONCl, 244.0524, found, 244.0530.
References:
Sarmiento, Gabriela P.;Vitale, Roxana G.;Afeltra, Javier;Moltrasio, Graciela Y.;Moglioni, Albertina G. [European Journal of Medicinal Chemistry,2011,vol. 46,# 1,p. 101 - 105] Location in patent:experimental part

86-74-8
347 suppliers
$10.00/5g
541-88-8
165 suppliers
$10.00/5g

38002-61-8
16 suppliers
$57.50/250mg