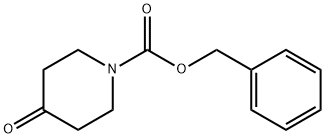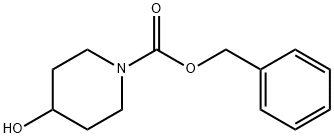
Benzyl 4-hydroxy-1-piperidinecarboxylate synthesis
- Product Name:Benzyl 4-hydroxy-1-piperidinecarboxylate
- CAS Number:95798-23-5
- Molecular formula:C13H17NO3
- Molecular Weight:235.28
5382-16-1

501-53-1

95798-23-5
General procedure for the synthesis of N-Benzyloxycarbonyl-4-hydroxypiperidine from 4-hydroxypiperidine and benzyl chloroformate: over 30 min, benzyl chloroformate (8.55 mL; 60 mmol) was slowly added dropwise to a well-stirred 4-hydroxypiperidine (5 g; 49.44 mmol), a 1N aqueous NaOH solution (60 mL; 60 mmol) and dioxane (60 mL) in a in a cooled mixture. After dropwise addition, the reaction mixture was continued to stir for 30 minutes. Upon completion of the reaction, the reaction mixture was treated with water and subsequently concentrated and acidified with hydrochloric acid to pH 2. The acidified aqueous phase was extracted with EtOAc, the organic phases were combined, washed with water and saturated brine in that order, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified by fast column chromatography (silica gel was the stationary phase, and the eluents were petroleum ether with 50% dichloromethane (60-80 °C) and 10% acetonitrile containing 2% methanol in chloroform solution). The final product was 12.5 g (99% yield) of N-Cbz-4-hydroxypiperidine as an oil; mass spectrum (chemical ionization): m/z 236 (M++1), 218, 192, 174, 91.

5382-16-1
0 suppliers
$9.00/5 g

501-53-1
408 suppliers
$10.00/1g

95798-23-5
136 suppliers
$5.00/250mg
5382-17-2
128 suppliers
$9.00/1g

501-53-1
408 suppliers
$10.00/1g

95798-23-5
136 suppliers
$5.00/250mg
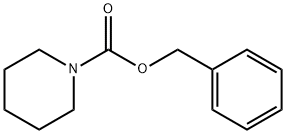
3742-91-4
21 suppliers
inquiry

95798-23-5
136 suppliers
$5.00/250mg
![1-Piperidinecarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, phenylmethyl ester](/CAS/20210305/GIF/97231-85-1.gif)
97231-85-1
0 suppliers
inquiry

95798-23-5
136 suppliers
$5.00/250mg