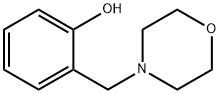
CHEMBRDG-BB 4022458 synthesis
- Product Name:CHEMBRDG-BB 4022458
- CAS Number:4438-01-1
- Molecular formula:C11H15NO2
- Molecular Weight:193.24

110-91-8
674 suppliers
$9.00/1g

50-00-0
840 suppliers
$10.00/25g

98-80-6
694 suppliers
$5.00/5g

4438-01-1
12 suppliers
$31.00/200μmol
Yield:4438-01-1 94%
Reaction Conditions:
with tert.-butylhydroperoxide in dichloromethane;water at 80; for 8 h;Sealed tube;regioselective reaction;Mannich Aminomethylation;Concentration;Reagent/catalyst;Solvent;Temperature;
Steps:
Typical procedure for the synthesis of Mannich bases
General procedure: In a typical experiment, phenylboronic acid (0.5 mmol), paraformaldehyde (0.5 mmol), morpholine (0.5 mmol), TBHP (1.5 equiv) and CH2Cl2 (2 mL) were added into a sealed tube. Subsequently, the reaction was carried out at 80 °C under magnetic stirring for 8 h. After cooling down, the reaction mixture was diluted with ethyl acetate. Then, the mixture was concentrated under vacuum, and the crude residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (50:1) to afford the desired pure product.
References:
Liu, Juan;Yuan, Gaoqing [Tetrahedron Letters,2017,vol. 58,# 15,p. 1470 - 1473] Location in patent:supporting information

110-91-8
674 suppliers
$9.00/1g

50-00-0
840 suppliers
$10.00/25g

108-95-2
709 suppliers
$14.00/25g

4438-01-1
12 suppliers
$31.00/200μmol

7309-48-0
0 suppliers
inquiry

108-95-2
709 suppliers
$14.00/25g

4438-01-1
12 suppliers
$31.00/200μmol

110-91-8
674 suppliers
$9.00/1g
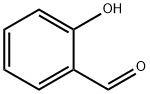
90-02-8
438 suppliers
$9.00/1g

4438-01-1
12 suppliers
$31.00/200μmol
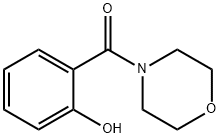
3202-84-4
17 suppliers
$28.60/10MG

4438-01-1
12 suppliers
$31.00/200μmol