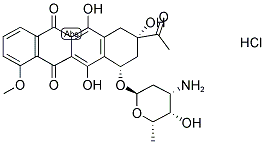
Daunorubicin hydrochloride synthesis
- Product Name:Daunorubicin hydrochloride
- CAS Number:23541-50-6
- Molecular formula:C27H29NO10.ClH
- Molecular Weight:563.98
![5,12-Naphthacenedione, 7,7'-[methylenebis[[(3aS,4S,6R,7aS)-tetrahydro-4-methyl-2H-pyrano[4,3-d]oxazole-1,6(6H)-diyl]oxy]]bis[9-acetyl-7,8,9,10-tetrahydro-6,9,11-trihydroxy-4-methoxy-, (7S,7'S,9S,9'S)-](/CAS/20210305/GIF/193743-47-4.gif)
193743-47-4
0 suppliers
inquiry

23541-50-6
502 suppliers
$15.00/10mg
Yield:-
Reaction Conditions:
Stage #1: daunoform in dimethylsulfoxide-d6;water-d2; for 24 h;
Stage #2: with hydrogenchloride in dimethylsulfoxide-d6;water-d2;
Steps:
6; 7
Crystalline dimeric formaldehyde conjugate of daunorubicin (2.8 mg, 2.6 μmol) was dissolved in 500 μL of DMSO-d6 (stored over 3 A molecular sieves) and analyzed by 400 MHz, 1H NMR. NMR analysis showed that the dimeric formaldehyde conjugate of daunorubicin was stable in DMSO for at least 14 h. Upon addition of 25 μL of D2O to the 500 μL DMSO-d6 sample, the dimeric formaldehyde conjugate of daunorubicin reacted over a 24 h period to form an equilibrium mixture consisting of 67% dimeric formaldehyde conjugate of daunorubicin and 32% of an intermediate. Because of the complexity of the spectrum, a structure could not be assigned to the intermediate; however, the intermediate appeared to have an oxazolidine ring. Upon addition of 3 equiv of hydrochloric acid in 130 μL of deuterium oxide, hydrolysis to daunorubicin-HCl was complete. The hydrochloric acid solution was prepared by the addition of 10 μL of concentrated HCl to 990 μL of D2O. The formaldehyde released from the dimeric formaldehyde conjugate of daunorubicin was detected as its hydrate. The final product was identified as daunorubicin by comparison of the NMR spectrum with that of a sample of daunorubicin plus 3 equiv of HCl. Further, the final product was isolated by removal of all volatile components under vacuum (0.1 Torr), and its 1H NMR spectrum was identical to that of daunorubicin-HCl.
References:
US6677309,2004,B1 Location in patent:Page/Page column 17; 18; 56
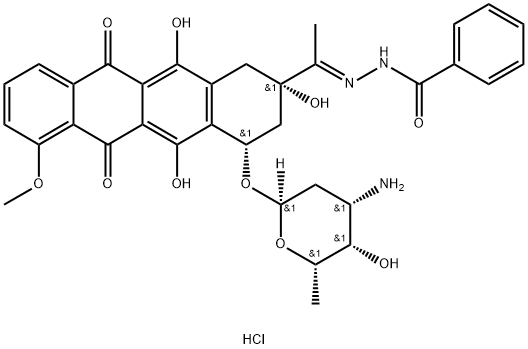
36508-71-1
34 suppliers
inquiry

23541-50-6
502 suppliers
$15.00/10mg
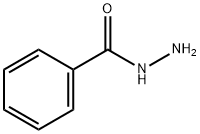
613-94-5
348 suppliers
$5.00/10g
![3-Pyridinecarboxylic acid, [1-[4-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl]ethylidene]hydrazide, monohydrochloride, (2S-cis)- (9CI)](/CAS/20211123/GIF/110925-43-4.gif)
110925-43-4
0 suppliers
inquiry
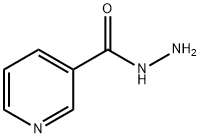
553-53-7
234 suppliers
$5.00/5g

23541-50-6
502 suppliers
$15.00/10mg
![4-Pyridinecarboxylic acid, [1-[4-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl]ethylidene]hydrazide, monohydrochloride, (2S-cis)- (9CI)](/CAS/20211123/GIF/110925-39-8.gif)
110925-39-8
0 suppliers
inquiry
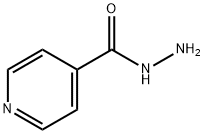
54-85-3
725 suppliers
$6.00/10g

23541-50-6
502 suppliers
$15.00/10mg
![Benzoic acid, 4-amino-, [1-[4-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl]ethylidene]hydrazide, monohydrochloride, (2S-cis)- (9CI)](/CAS/20211123/GIF/110925-32-1.gif)
110925-32-1
0 suppliers
inquiry

23541-50-6
502 suppliers
$15.00/10mg
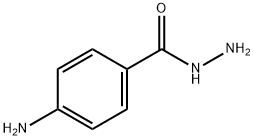
5351-17-7
156 suppliers
$10.00/1g