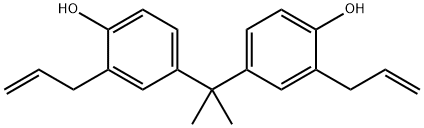
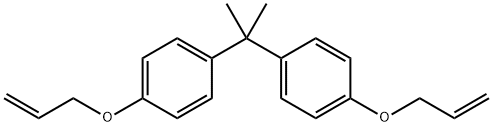
Diallyl bisphenol A synthesis
- Product Name:Diallyl bisphenol A
- CAS Number:1745-89-7
- Molecular formula:C21H24O2
- Molecular Weight:308.41
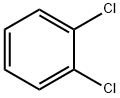
80-05-7
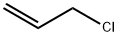
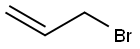
107-05-1

1745-89-7
To an autoclave equipped with a stirrer was added 356 g of ethylene glycol monobutyl ether and 19 g of water, followed by 150 g (0.657 mol) of bisphenol A. Sequentially, 53 g (1.325 mol) of sodium hydroxide and 14 g (0.132 mol) of sodium carbonate were added. Next, 121 g (1.581 mol) of 3-chloropropene was added, the autoclave was sealed, and the reaction was heated at 100 to 105 °C for 8 hours. Upon completion of the reaction, unreacted 3-chloropropene was removed by distillation according to the same method as in Example 1 and aqueous phase separation was performed. After neutralizing the reaction mixture, heat treatment was performed to obtain a glycol monobutyl ether solution of bisphenol A diallyl ether. The solution had a sodium ion content of about 10 ppm. 2,2-bis(4-allylhydroxyphenyl)propane was analyzed by HPLC at a composition ratio of 95.0% (area percentage), bisphenol A at 0.2%, and 4-hydroxyphenyl-4'-allylhydroxyphenylpropane (bisphenol A monoallyl ether) at 2.2%. Based on the content of bisphenol A monoallyl ether, a yield of 95% was calculated. The thermally filtered reaction solution (filtrate) was transferred to an autoclave and heated and reacted under closed conditions (0.1 MPaG) at 195-200 °C for 7 hours. Subsequently, ethylene glycol monobutyl ether was removed by distillation under reduced pressure at 150 °C to give 166 g of 4,4'-(propane-2,2-diyl)bis(2-allylphenol) (yield: 82%). The HPLC composition ratio of the resulting product was 92.0% (% area).

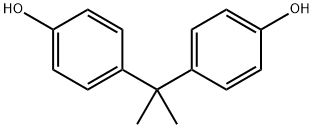
80-05-7
708 suppliers
$13.00/100g
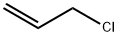
107-05-1
424 suppliers
$16.80/100ML

1745-89-7
219 suppliers
$6.00/10g
Yield:1745-89-7 82%
Reaction Conditions:
with sodium carbonate;sodium hydroxide in water at 100 - 105; for 8 h;Autoclave;
Steps:
3 Example 3
To an autoclave equipped with a stirrer, 356 g of ethylene glycol monobutyl ether and 19 g of water were added 150 g (0.657 mol) of 2,2'-bis (4-hydroxyphenyl) propane (hereinafter referred to as bisphenol A), 53 g (1.325 mol) of sodium hydroxide and 14 g (0.132 mol) of sodium carbonate were added in sequence. Subsequently, 121 g (1.581 mol) of allyl chloride was added and the mixture was reacted by heating at 100 to 105 DEG C for 8 hours while keeping the mixture in a sealed state. After the completion of the reaction, allyl chloride was distilled off in the same manner as in Example 1, and a water separation was performed. After neutralization, thermal treatment was performed to obtain an ethylene glycol monobutyl ether solution of bisphenol A diallyl ether. The reaction solution had an Na content of about 10 ppm. The HPLC composition ratio (area percentage) was 95.0% for 2,2-bis (4-allyl hydroxyphenyl) propane, 0.2% for bisphenol A, and 4-hydroxyphenyl-4'-allyl hydroxyphenylpropane Bisphenol A monoallyl ether) was 2.2%. The yield calculated from the content of bisphenol A diallyl ether was 95%.The reaction solution (filtrate) after the thermal filtration was injected into an autoclave and the heating potential reaction was carried out at 195 to 200 in a closed state (0.1 MPaG) for 7 hours. Thereafter, ethylene glycol monobutyl ether was distilled off under reduced pressure at 150 to obtain 166 g of 2,2'-bis (3-allyl-4-hydroxyphenyl) propane (hereinafter referred to as diallyl-bisphenol A) Yield: 82%). The obtained diallyl-bisphenol A had a HPLC composition ratio (area percentage) of 92.0%.
References:
KR101597659,2016,B1 Location in patent:Paragraph 0104-0107

80-05-7
708 suppliers
$13.00/100g

1745-89-7
219 suppliers
$6.00/10g
3739-67-1
152 suppliers
$73.00/100mg

1745-89-7
219 suppliers
$6.00/10g

80-05-7
708 suppliers
$13.00/100g

557-40-4
80 suppliers
$63.00/25mL
![Phenol, 4-[1-(4-hydroxyphenyl)-1-methylethyl]-2-(2-propen-1-yl)-](/CAS/20210305/GIF/109348-07-4.gif)
109348-07-4
0 suppliers
inquiry

1745-89-7
219 suppliers
$6.00/10g
![Phenol, 4-[1-methyl-1-[4-(2-propen-1-yloxy)phenyl]ethyl]-2-(2-propen-1-yl)-](/CAS/20210305/GIF/105577-85-3.gif)
105577-85-3
0 suppliers
inquiry

59501-41-6
24 suppliers
inquiry

3739-67-1
152 suppliers
$73.00/100mg