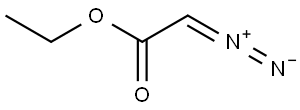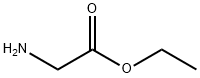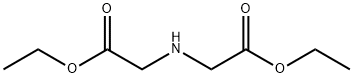
Diethyl iminodiacetate synthesis
- Product Name:Diethyl iminodiacetate
- CAS Number:6290-05-7
- Molecular formula:C8H15NO4
- Molecular Weight:189.21

64-17-5
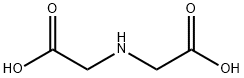
142-73-4

6290-05-7
Ethanol was placed in a reaction flask at 0°C and stirred, thionyl chloride was added slowly and dropwise, and stirring was maintained until the temperature of the reaction mixture was raised to room temperature (about 30 min). Subsequently, iminodiacetic acid (3.33 g, 25 mmol) was added to the reaction system and the temperature of the reaction was raised to reflux and the reaction was continued overnight. Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched with saturated sodium bicarbonate solution (20 mL) and the pH was adjusted to neutral by adding solid sodium bicarbonate. After distillation under reduced pressure to remove most of the ethanol, the mixture was extracted three times with ethyl acetate (3 x 20 mL). The organic phases were combined, washed once with brine, dried through magnesium sulfate and filtered. Finally, the target product diethyl iminodiacetate (4.15 g, 88% yield) was obtained by distillation under reduced pressure (recovery of organic solvent).

142-73-4
439 suppliers
$6.00/25g

6290-05-7
232 suppliers
$27.00/50g
Yield:-
Reaction Conditions:
with thionyl chloride in ethanol;dichloromethane;water;N,N-dimethyl-formamide
Steps:
1.A Step A
Step A Synthesis of Iminodiacetic Acid Diethyl Ester A stirred solution of 931.0 grams (7.0 moles) of iminodiacetic acid and 1.0 ml of N,N-dimethylformamide in 5.25 liters of ethanol was cooled to 12° C. During a 1.5 hour period 2,100 grams (17.65 moles) of thionyl chloride was added dropwise to the cold solution. After complete addition the reaction mixture was heated at 60° C. for three hours, at reflux for two hours, then allowed to stand at room temperature for approximately 16 hours. The mixture was distilled at reduced pressure to remove 4.0 liters of ethanol leaving a liquid residue. The residue was dissolved in 4.0 liters of methylene chloride, then extracted with 3.5 liters of an aqueous 20% sodium carbonate solution. The aqueous extract was back washed with 1.0 liter of methylene chloride. The organic phases were combined, washed with 1.0 liter of water and evaporated under reduced pressure to leave a liquid. The liquid was purified by distillation under reduced pressure to yield 880.0 grams of iminodiacetic acid diethyl ester (b.p. 123° C./4.5 mmHg).
References:
FMC Corporation US4389528, 1983, A

64-17-5
825 suppliers
$10.00/50g

142-73-4
439 suppliers
$6.00/25g

6290-05-7
232 suppliers
$27.00/50g
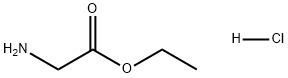
623-33-6
657 suppliers
$5.00/10g
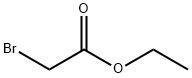
105-36-2
349 suppliers
$5.00/5 g

6290-05-7
232 suppliers
$27.00/50g

64-17-5
825 suppliers
$10.00/50g
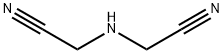
628-87-5
204 suppliers
$13.43/1gm:

6290-05-7
232 suppliers
$27.00/50g