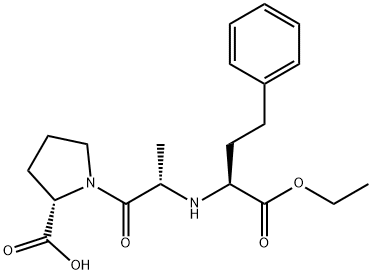
Enalapril synthesis
- Product Name:Enalapril
- CAS Number:75847-73-3
- Molecular formula:C20H28N2O5
- Molecular Weight:376.45
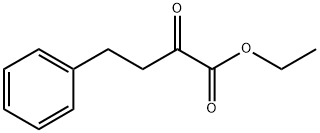
64920-29-2
255 suppliers
$10.00/1g
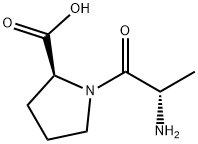
13485-59-1
215 suppliers
$13.00/250mg

75847-73-3
186 suppliers
$30.00/500mg
Yield:75847-73-3 70%
Reaction Conditions:
with hydrogen in ethanol at 45; under 735.074 - 772.577 Torr;Catalytic behavior;Kinetics;Solvent;Reagent/catalyst;
Steps:
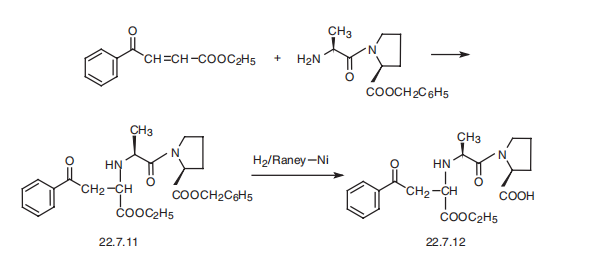
Procedure for hydrogenation N-alkylation of α-alanyl-α-proline with ethyl 2-oxo-4-phenylbutanoate.
A weighed sample of the catalyst (100-500 mg) was loaded under a layer of solvent (50 mL) in a glass reactor equipped with a jacket for thermo stating and a magnetic stirrer for stirring in a fl ow of hydrogen. The catalyst was activated with hydrogen for 20-30 min. Then, α-alanyl-α-proline (4 g, 0.022 mol) and ethyl 2-oxo-4-phenylbutanoate (10 g, 0.043 mol) were introduced into the reactor in a fl ow of hydrogen. The reaction mixtures were stirred at a constant rate of 900-1100 rpm at a hydrogen pressure of 98-103 kPa. After the reaction completion, the mixture was fi ltered and concentrated by evaporation to give a pale yellow oily residue (6.0 g). Then, the oily residue was dispersed in a solution of sodium chloride (20 g) in water (100 mL), the pH of which was adjusted to 8.5 with K2HPO4, and extracted with ethyl acetate (2×100 mL). The aqueous solution was acidifi ed with 1 M H3PO4 to pH 4.2 and again extracted with ethyl acetate (4×100 mL). The extract was dried with anhydrous sodium sulfate and concentrated to obtain an oily substance (3.30 g). Then, the residue of the oily substance was dissolved in warm water (100 mL, 60 C) and fi ltered. Upon cooling the fi ltrate, a white amorphous substance of enalapril is formed (the highest yield on the AV-17-8-Pd catalyst was 2.63 g, 70 wt.%). The mass yields for all the studied catalysts are given in Tables 1 and 2 and ranging from 30 to 70 wt.%. The purity of the obtained enalapril, as determined by TLC, was no less than 98.0%.
References:
Abdullaev, M. G. [Russian Chemical Bulletin,2020,vol. 69,# 10,p. 1923 - 1927][Izv. Akad. Nauk, Ser. Khim.,2020,vol. 69,# 10,p. 1923 - 1927,5]
![L-Proline, N-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-, phenylmethyl ester](/CAS/20210305/GIF/120924-94-9.gif)
120924-94-9
0 suppliers
inquiry

75847-73-3
186 suppliers
$30.00/500mg
![N-[(S)-(+)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanine](/CAS/GIF/82717-96-2.gif)
82717-96-2
331 suppliers
$6.00/5g
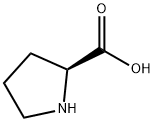
147-85-3
1058 suppliers
$6.00/25g

75847-73-3
186 suppliers
$30.00/500mg
![Benzenebutanoic acid, α-[[(1S)-2-[(2,5-dioxo-1-pyrrolidinyl)oxy]-1-methyl-2-oxoethyl]amino]-, ethyl ester, (αS)-](/CAS/20210305/GIF/89371-34-6.gif)
89371-34-6
1 suppliers
inquiry

147-85-3
1058 suppliers
$6.00/25g

75847-73-3
186 suppliers
$30.00/500mg

64920-29-2
255 suppliers
$10.00/1g

13485-59-1
215 suppliers
$13.00/250mg

75847-73-3
186 suppliers
$30.00/500mg
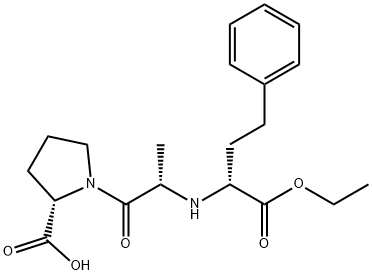
76420-74-1
26 suppliers
inquiry