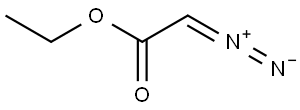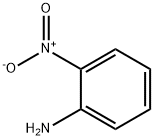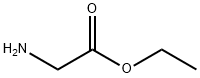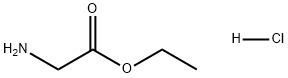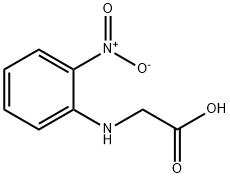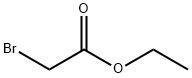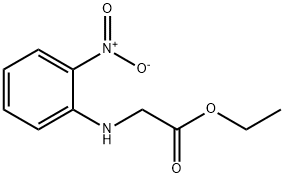
Ethyl 2-[(2-nitrophenyl)amino]acetate synthesis
- Product Name:Ethyl 2-[(2-nitrophenyl)amino]acetate
- CAS Number:5428-05-7
- Molecular formula:C10H12N2O4
- Molecular Weight:224.21
Yield:5428-05-7 92%
Reaction Conditions:
with 1-methylimidazolium tetrafluoroborate in neat (no solvent) at 20; for 2 h;
Steps:
General procedure for the N-H insertion reaction
General procedure: To a mixture of amine (2 mmol) and ethyl diazoacetate (see Table 1 for equivalents), [Hmim][BF4] (10 mol %) was added. The mixture was stirred at room temperature until the completion of the reaction, as indicated by TLC. Next, H2O and CH2Cl2 were added. The mixture was decanted, the products being soluble in CH2Cl2. The ionic liquid being soluble in H2O was isolated after drying under vacuum and was reused in subsequent reactions.
References:
Akbari, Jafar;Ebrahimi, Ali;Heydari, Akbar [Tetrahedron Letters,2014,vol. 55,# 40,p. 5417 - 5419]